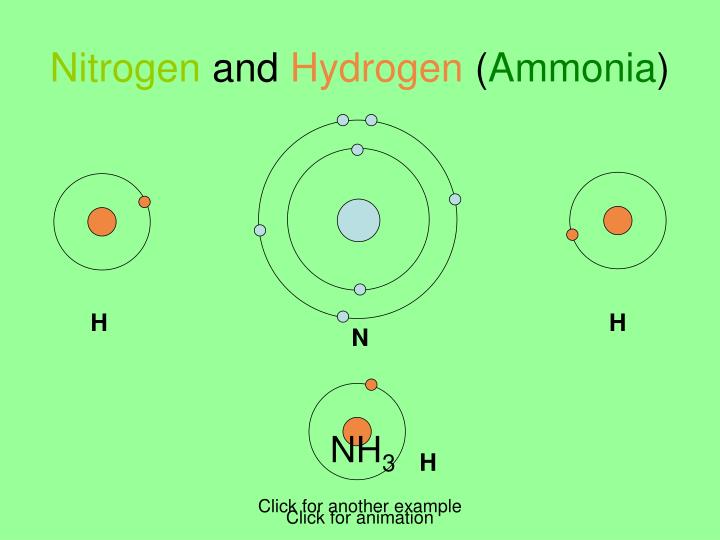
Hydrogen Gas And Nitrogen Gas React To Form Ammonia Gas.
Hydrogen Gas And Nitrogen Gas React To Form Ammonia Gas. - Thus, 7.7 ml = 0.0077 l. N₂ (g) + 3 h₂ (g) → 2 nh₃ (g) the. 1 n2 (s) + 3 h2 (g) → 2 nh3 (g)what mass of nitrogen is required to completely react with 800.0 ml. Web nitrogen and hydrogen combine to form ammonia via the following reaction: What volume of ammonia would be produced by this reaction if 8.8 m² of nitrogen were consumed? Web nitrogen and hydrogen gases react to form ammonia gas as follows: Web hydrogen gas and nitrogen gas react to form ammonia gas. Web chemistry chemistry questions and answers hydrogen gas reacts with nitrogen gas to form ammonia gas write a balanced chemical equation for this reaction ローロ x ?. Web in a chemical reaction the starting materials are treated as reactants. H 2 ( g) hydrogen + n 2 ( g) nitrogen ⇌ nh 3 ( g).
Web 1 day agofortunately, ammonia is a promising hydrogen carrier that can be easily liquified under milder conditions, transported, and decomposed with the help of a catalyst. $$ \mathrm{n}_{2}(g)+3 \mathrm{h}_{2}(g) \longrightarrow 2 \mathrm{nh}_{3}(g) $$ at a. Web in a chemical reaction the starting materials are treated as reactants. Extra content n2(g) + 3h2(g). N₂ (g) + 3 h₂ (g) → 2 nh₃ (g) the. Web how many moles of ammonia gas are produced in the following reaction if 25 moles of nitrogen gas reacts with hydrogen? What volume of ammonia would be produced by this reaction if 5.53 ml of hydrogen. Web nitrogen and hydrogen gases react to form ammonia gas as follows: Web nitrogen gas and hydrogen gas react to form ammonia gas via the folowing reaction. Web nitrogen gas reacts with hydrogen gas to form ammonia gas.
Web how many moles of ammonia gas are produced in the following reaction if 25 moles of nitrogen gas reacts with hydrogen? Web in a chemical reaction the starting materials are treated as reactants. What volume of ammonia would be produced by this reaction if 8.8 m² of nitrogen were consumed? Web chemistry chemistry questions and answers hydrogen gas reacts with nitrogen gas to form ammonia gas write a balanced chemical equation for this reaction ローロ x ?. In the chemical equation above, 3 moles of hydrogen gas react with 1 mole of nitrogen gas to produce 2 moles of ammonia gas. Hydrogen gas and nitrogen gas react to form ammonia gas. H 2 ( g) hydrogen + n 2 ( g) nitrogen ⇌ nh 3 ( g). Web nitrogen gas reacts with hydrogen gas to form ammonia gas. In the reaction of nitrogen gas, n2, with hydrogen gas, h2, to form ammonia gas, nh3, how many moles of hydrogen are needed to react with two moles of. N₂ (g) + 3 h₂ (g) → 2 nh₃ (g) the.
Solved 10 g of nitrogen is reacted with 5.0 g of hydrogen to
Web how many moles of ammonia gas are produced in the following reaction if 25 moles of nitrogen gas reacts with hydrogen? The volume of nitrogen (n₂) gas = 7.7 ml. In the reaction of nitrogen gas, n2, with hydrogen gas, h2, to form ammonia gas, nh3, how many moles of hydrogen are needed to react with two moles of..
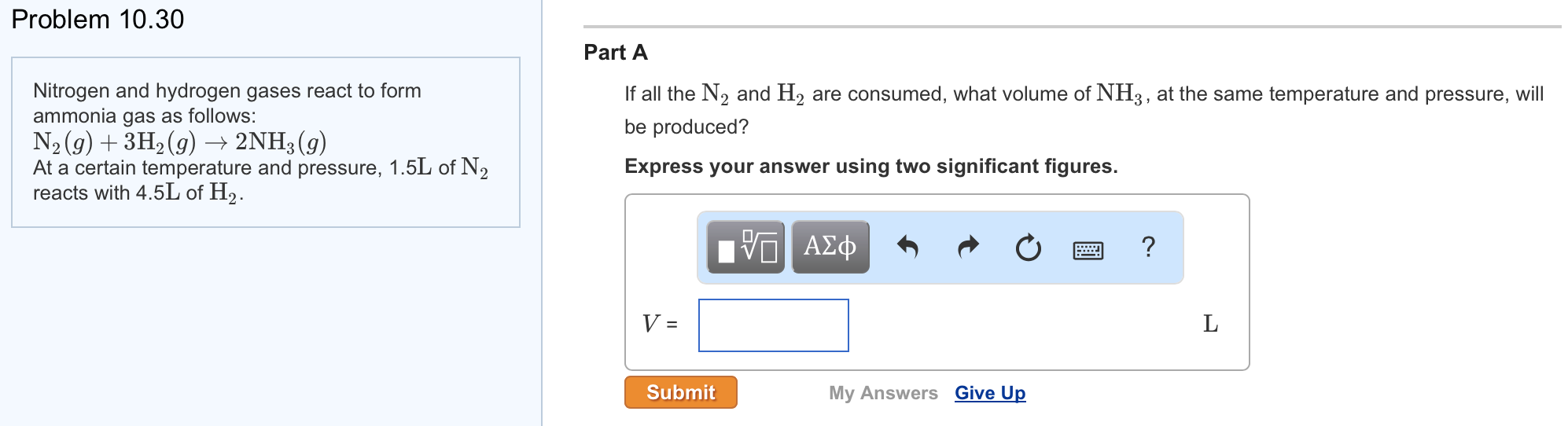
Solved Nitrogen And Hydrogen Gases React To Form Ammonia
1 n2 (s) + 3 h2 (g) → 2 nh3 (g)what mass of nitrogen is required to completely react with 800.0 ml. Web hydrogen gas and nitrogen gas react to form ammonia gas. Nitrogen and hydrogen gases react to form ammonia gas via the following reaction: The volume of nitrogen (n₂) gas = 7.7 ml. Web the following chemical reaction.
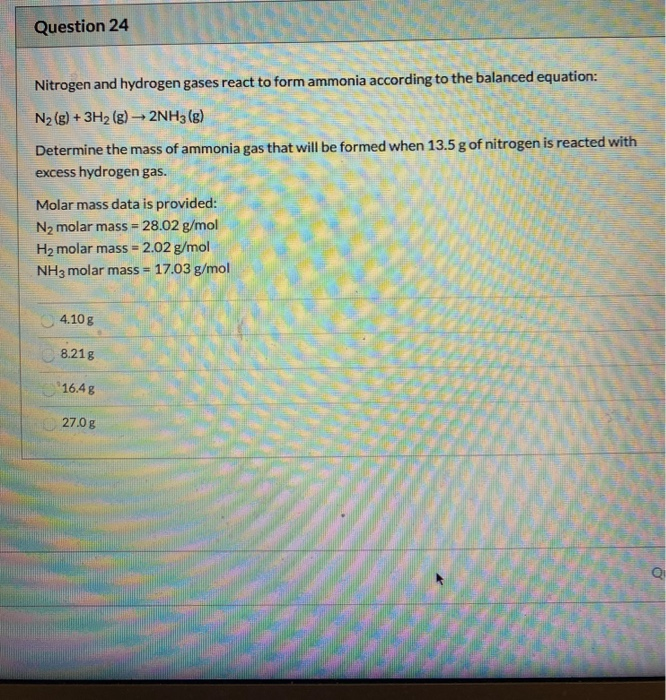
Solved Question 24 Nitrogen and hydrogen gases react to form
What volume of ammonia would be produced by this reaction if 5.53 ml of hydrogen. Web how many moles of ammonia gas are produced in the following reaction if 25 moles of nitrogen gas reacts with hydrogen? Hydrogen gas and nitrogen gas react to form ammonia gas. Web the balanced equation for the reaction is: In the chemical equation above,.
Solved Nitrogen (N2) gas and hydrogen (H2) gas react to form
N2 (g)+3h2 (g)↽−−⇀2nh3 (g) when 0.600 mol n2 and 0.400 mol h2 are placed in a 1.0. Web chemistry chemistry questions and answers hydrogen gas reacts with nitrogen gas to form ammonia gas write a balanced chemical equation for this reaction ローロ x ?. What volume of ammonia would be produced by this reaction if 5.53 ml of hydrogen. Hydrogen.
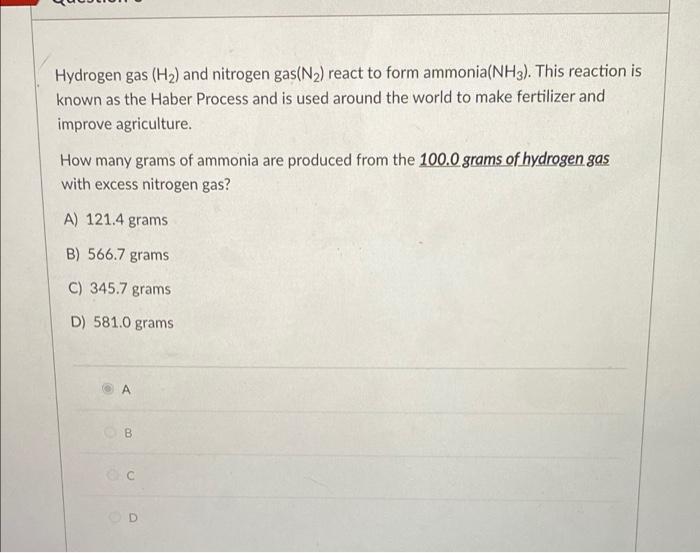
Solved Hydrogen gas (H2) and nitrogen gas(N2) react to form
Web nitrogen and hydrogen gases react to form ammonia gas as follows: $$ \mathrm{n}_{2}(g)+3 \mathrm{h}_{2}(g) \longrightarrow 2 \mathrm{nh}_{3}(g) $$ at a. In the chemical equation above, 3 moles of hydrogen gas react with 1 mole of nitrogen gas to produce 2 moles of ammonia gas. 1 ml = 0.001 l. In the reaction of nitrogen gas, n2, with hydrogen gas,.
D Question 9 1 pts Nitrogen and hydrogen gas react to form ammonia (NH3
N2 (nitrogen) + 3h2 (hydrogen) <=> 2nh3 (ammonia),. What volume of ammonia would be produced by this reaction if 5.53 ml of hydrogen. $$ \mathrm{n}_{2}(g)+3 \mathrm{h}_{2}(g) \longrightarrow 2 \mathrm{nh}_{3}(g) $$ at a. N2 (g) + 3h2 (g) +2nh3 (g) at a certain temperature and pressure, 1.1 l of n2. 1 ml = 0.001 l.
PPT Reaction Rates PowerPoint Presentation, free download ID6516140
1 ml = 0.001 l. Web in a chemical reaction the starting materials are treated as reactants. 1 n2 (s) + 3 h2 (g) → 2 nh3 (g)what mass of nitrogen is required to completely react with 800.0 ml. Web how many moles of ammonia gas are produced in the following reaction if 25 moles of nitrogen gas reacts with.
[Best Answer] hydrogen gas combines with nitrogen to form Ammonia
What volume of ammonia would be produced by this reaction if 5.53 ml of hydrogen. N2 (g) + 3h2 (g) +2nh3 (g) at a certain temperature and pressure, 1.1 l of n2. N₂ (g) + 3 h₂ (g) → 2 nh₃ (g) the. Web the balanced equation for the reaction is: H 2 ( g) hydrogen + n 2 (.
Nitrogen gas and hydrogen gas combine to form gaseous ammonia
Web the balanced equation for the reaction is: H 2 ( g) hydrogen + n 2 ( g) nitrogen ⇌ nh 3 ( g). N2 (nitrogen) + 3h2 (hydrogen) <=> 2nh3 (ammonia),. Web hydrogen gas and nitrogen gas react to form ammonia gas. What volume of ammonia would be produced by this reaction if 5.53 ml of hydrogen.
PPT Covalent Bonding PowerPoint Presentation ID5648526
Web 1 day agofortunately, ammonia is a promising hydrogen carrier that can be easily liquified under milder conditions, transported, and decomposed with the help of a catalyst. Web nitrogen gas reacts with hydrogen gas to form ammonia gas. N2 (nitrogen) + 3h2 (hydrogen) <=> 2nh3 (ammonia),. Web nitrogen and hydrogen combine to form ammonia via the following reaction: N₂ (g).
What Volume Of Ammonia Would Be Produced By This Reaction If 8.8 M² Of Nitrogen Were Consumed?
Extra content n2(g) + 3h2(g). Nitrogen and hydrogen gases react to form ammonia gas via the following reaction: Web in a chemical reaction the starting materials are treated as reactants. N2 (nitrogen) + 3h2 (hydrogen) <=> 2nh3 (ammonia),.
N₂ (G) + 3 H₂ (G) → 2 Nh₃ (G) The.
Web nitrogen and hydrogen gases react to form ammonia gas as follows: Web how many moles of ammonia gas are produced in the following reaction if 25 moles of nitrogen gas reacts with hydrogen? N2 (g) + 3h2 (g) +2nh3 (g) at a certain temperature and pressure, 1.1 l of n2. Web nitrogen and hydrogen combine to form ammonia via the following reaction:
1 Ml = 0.001 L.
1 n2 (s) + 3 h2 (g) → 2 nh3 (g)what mass of nitrogen is required to completely react with 800.0 ml. N2 (g)+3h2 (g)↽−−⇀2nh3 (g) when 0.600 mol n2 and 0.400 mol h2 are placed in a 1.0. Web nitrogen gas and hydrogen gas react to form ammonia gas via the folowing reaction. Hydrogen gas and nitrogen gas react to form ammonia gas.
In The Reaction Of Nitrogen Gas, N2, With Hydrogen Gas, H2, To Form Ammonia Gas, Nh3, How Many Moles Of Hydrogen Are Needed To React With Two Moles Of.
$$ \mathrm{n}_{2}(g)+3 \mathrm{h}_{2}(g) \longrightarrow 2 \mathrm{nh}_{3}(g) $$ at a. Web hydrogen gas and nitrogen gas react to form ammonia gas. Web chemistry chemistry questions and answers hydrogen gas reacts with nitrogen gas to form ammonia gas write a balanced chemical equation for this reaction ローロ x ?. What volume of ammonia would be produced by this reaction if 5.53 ml of hydrogen.



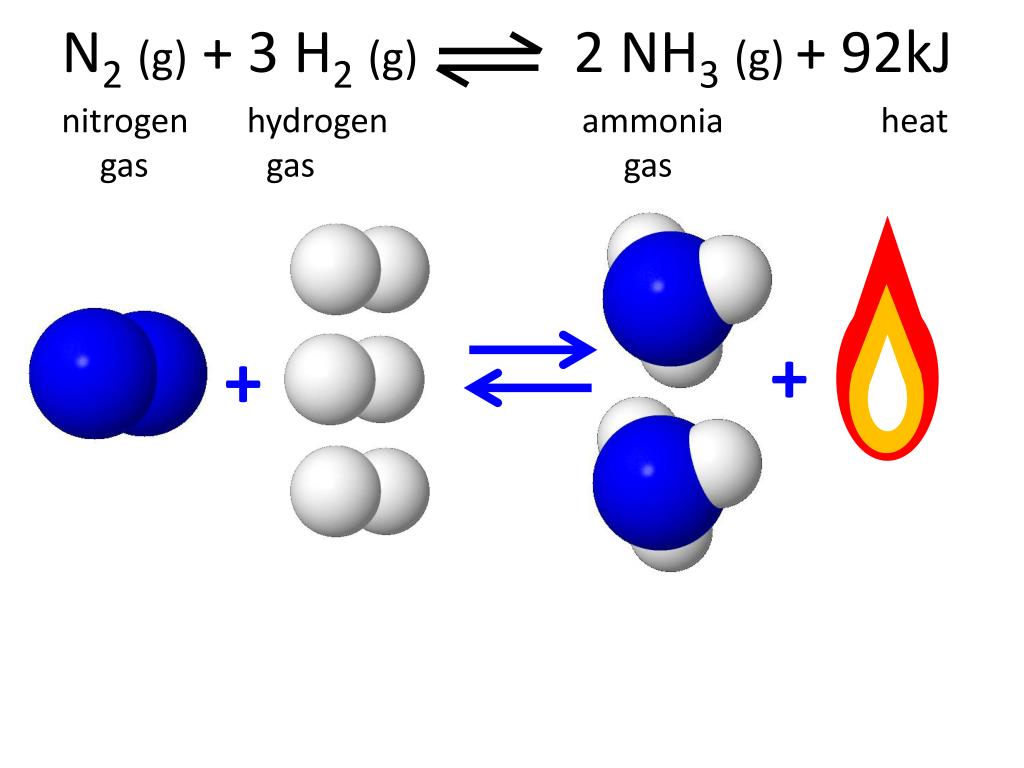
![[Best Answer] hydrogen gas combines with nitrogen to form Ammonia](https://hi-static.z-dn.net/files/dc1/9e1c135271e8d11c1d9621fa70f7ea35.jpg)