Barium Electron Configuration Long Form
Barium Electron Configuration Long Form - The number of the principal quantum shell, n, the letter that designates the orbital type (the subshell, l ), and 1s 2 2s 2 2p 1: The first two groups (columns) of the periodic table represent the 's' orbital group. In this video we'll use the periodic. It is also in the 2nd group (column) of the periodic table. The 6th row, s block, 2nd column. In this video we will write the electron configuration for ba 2+, the barium ion. Electron configuration can be done in two ways. The electron configuration of barium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 6s 2, if the electron arrangement is through orbitals. 1s 2 2s 2 2p 3:
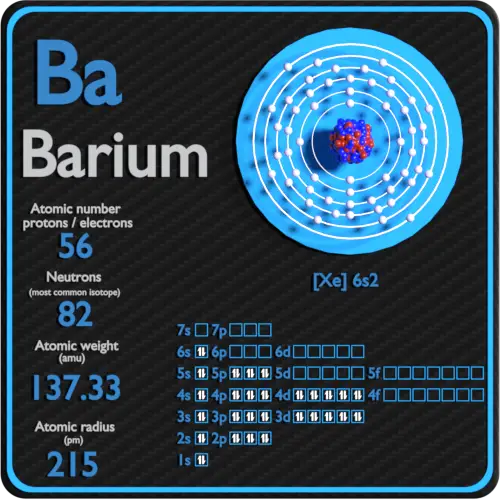
1000 k (727 °c, 1341 °f) boiling point: Electron configuration of nitrogen (n) [he] 2s 2 2p 3: The first two groups (columns) of the periodic table represent the 's' orbital group. Web how to write the electron configuration for barium. The chemical symbol of barium is ba. Web full electron configuration of barium: A horizontal row in the periodic table. We’ll also look at why barium forms a 2+ ion and how the electron. This means that the s,p,d,f electron configuration for barium must end with 6s^2. `1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 3d^10, 4s^2, 4p^6, 4d^10, 5s^2, 5p^6, 6s^2` thus, we can see that s, p.
Web electron configuration of barium is [xe] 6s2. Web electron configuration of barium. Web the commonly used long form of the periodic table is designed to emphasize electron configurations. The chemical element that belongs to the periodic table is called barium, its atomic number is 56 and it is represented by the symbol ba. Ba (barium) is an element with position number 56 in the periodic table. In this video we will write the electron configuration for ba 2+, the barium ion. This configuration shows that barium has six energy levels, and each level is filled with electrons. `1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 3d^10, 4s^2, 4p^6, 4d^10, 5s^2, 5p^6, 6s^2` thus, we can see that s, p. Electron configuration of oxygen (o. The 6th row, s block, 2nd column.
Barium Periodic Table and Atomic Properties
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2 cesium ← barium → lanthanum Web the atomic number of element barium is 56 and its electronic configuration can be written as: 1s 2 2s 2 2p 2: In this video we'll use the periodic. Web barium can be found in the 6th energy level (row) of the.
Electron Configuration Of Barium cloudshareinfo
Web the electron configuration of ba includes a total of 56 electrons. Electron configuration of boron (b) [he] 2s 2 2p 1: Web full electron configuration of barium: `1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 3d^10, 4s^2, 4p^6, 4d^10, 5s^2, 5p^6, 6s^2` thus, we can see that s, p. For each atom the subshells are given first in concise form, then with.
Barium electron configuration Newton Desk
Number of neutrons (most common/stable nuclide): The electron configuration of barium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. A horizontal row in the periodic table. Electron configuration of boron (b) [he] 2s 2 2p 1: Web the long form electron configuration of barium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2.
Ba 2+ Electron Configuration (Barium Ion) YouTube
Number of neutrons (most common/stable nuclide): The atomic number of each element increases by one, reading from left to right. Web electron configuration of barium. Web a vertical column in the periodic table. `1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 3d^10, 4s^2, 4p^6, 4d^10, 5s^2, 5p^6, 6s^2` thus, we can see that s, p.
A stepbystep description of how to write the electron configuration
`1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 3d^10, 4s^2, 4p^6, 4d^10, 5s^2, 5p^6, 6s^2` thus, we can see that s, p. In this video we'll use the periodic. 1s 2 2s 2 2p 2: Electron configuration of oxygen (o. Web the long form electron configuration of barium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2.
Symbol and electron diagram for barium Royalty Free Vector
Web the commonly used long form of the periodic table is designed to emphasize electron configurations. Electron configuration can be done in two ways. Web barium can be found in the 6th energy level (row) of the periodic table. Web electron configurations of the elements (data page) this page shows the electron configurations of the neutral gaseous atoms in their.
Atomic structure of oxygen stock illustration. Illustration of physics
1s 2 2s 2 2p 2: The atomic number of each element increases by one, reading from left to right. Possible oxidation states are +2. Web the commonly used long form of the periodic table is designed to emphasize electron configurations. The 6th row, s block, 2nd column.
Yeezy Quantum Barium Takes After a Chemical Element & 3 Other Yeezys!
Electron configuration of boron (b) [he] 2s 2 2p 1: Web an uncharged barium atom would have 56 electrons also. Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. The electron configuration of barium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2. 1s 2 2s.
Electron Configuration Of Barium cloudshareinfo
1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 10 5s 2 p 6 6s 2. Electronic configuration of the barium atom in ascending order of orbital energies: Electron configuration of oxygen (o. Electron configuration of boron (b) [he] 2s 2 2p 1: Web full electron configuration of barium:
56. Barium Quicycle Society
The atomic number of barium is 56and it belongs to the sixth period and the second group of the periodic table. 1000 k (727 °c, 1341 °f) boiling point: Electron configuration of nitrogen (n) [he] 2s 2 2p 3: When we write the configuration, we'll put all 56 electrons in orbitals around the nucleus of the cobalt atom. 2118 k.
We’ll Also Look At Why Barium Forms A 2+ Ion And How The Electron.
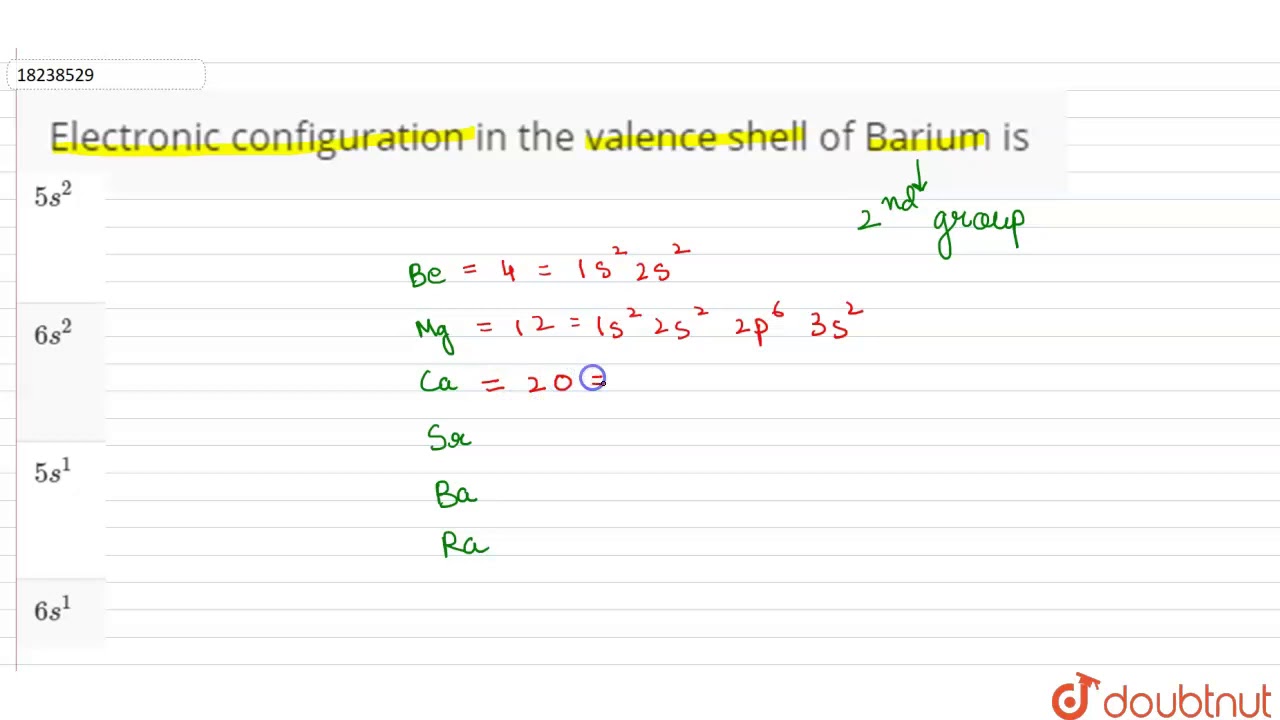
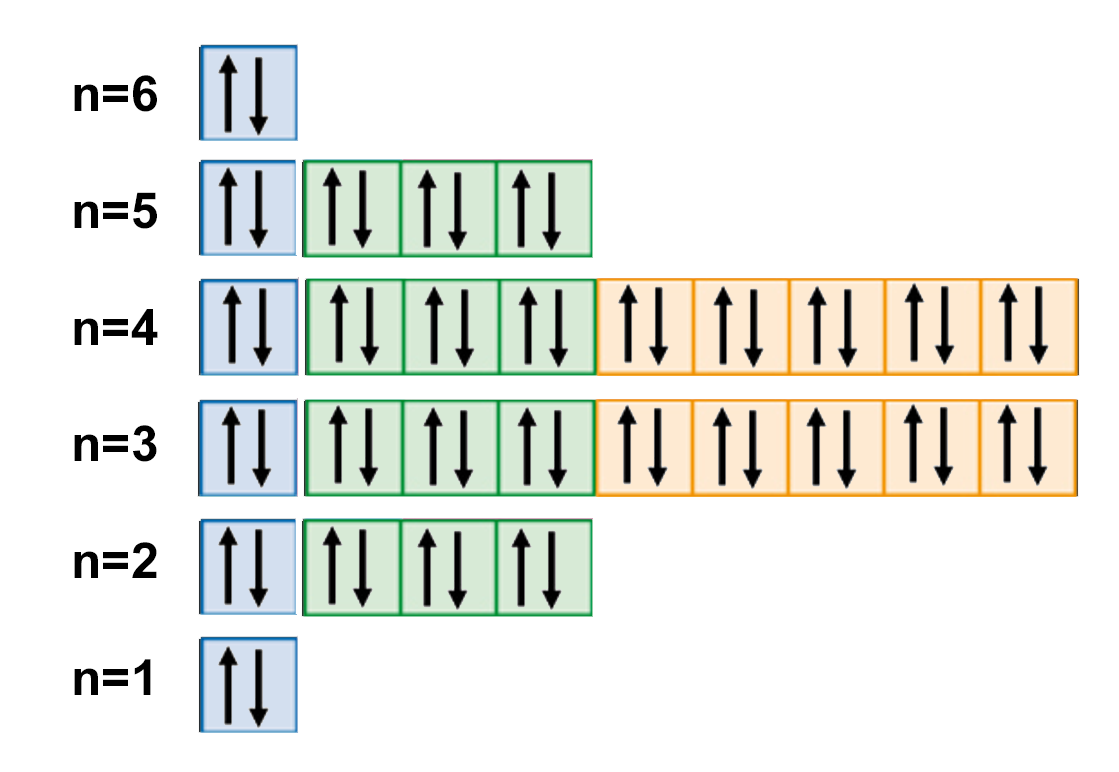
Web we can write the electron configuration of barium using four different methods: Web the swedish chemist carl wilhelm scheele discovered (1774) a new base (baryta, or barium oxide, bao) as a minor constituent in pyrolusite, and from that base he prepared some crystals of barium sulfate, which he sent to johan gottlieb gahn, the discoverer of manganese. 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 10 5s 2 p 6 6s 2. The first two groups (columns) of the periodic table represent the 's' orbital group.
Web Electron Configuration Of Barium Is [Xe] 6S2.
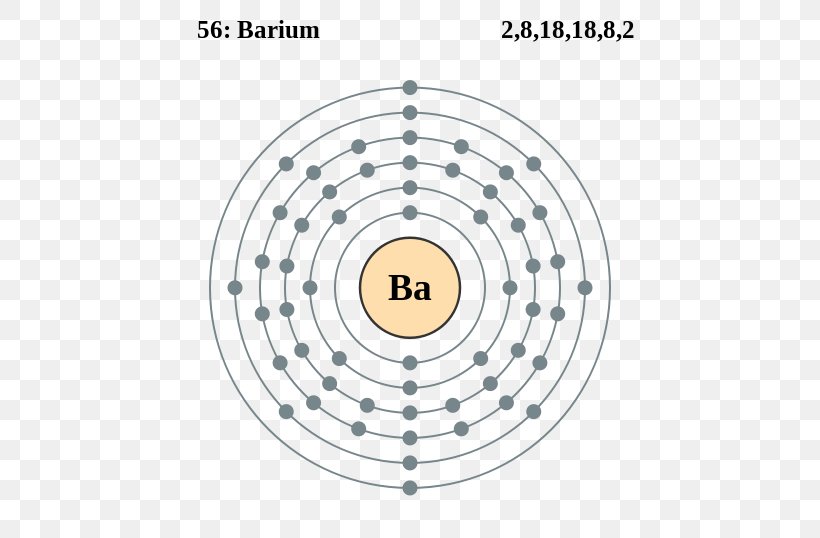
Electronic configuration of the barium atom in ascending order of orbital energies: 2, 8, 18, 18, 8, 2: A horizontal row in the periodic table. 1s 2 2s 2 2p 3:
The Number Of The Principal Quantum Shell, N, The Letter That Designates The Orbital Type (The Subshell, L ), And
1000 k (727 °c, 1341 °f) boiling point: In this video we'll use the periodic. Web the arrangement of electrons in barium in specific rules in different orbits and orbitals is called the electron configuration of barium. When we write the configuration, we'll put all 56 electrons in orbitals around the nucleus of the cobalt atom.
For Each Atom The Subshells Are Given First In Concise Form, Then With All Subshells Written Out, Followed By The Number Of Electrons Per Shell.
Number of neutrons (most common/stable nuclide): Web how to write the electron configuration for barium. The electron configuration of barium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 6s 2, if the electron arrangement is through orbitals. Located in the vi period.