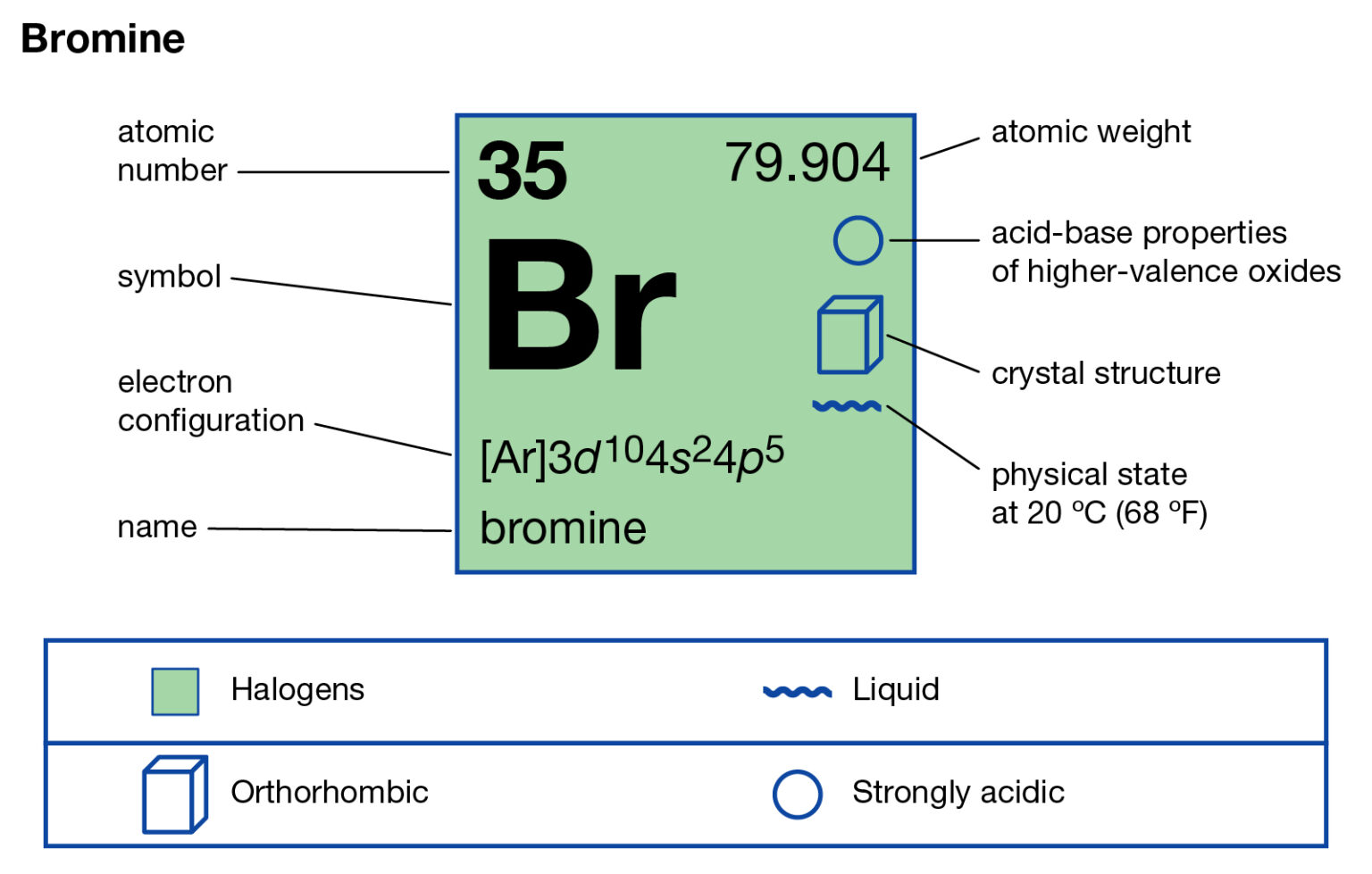
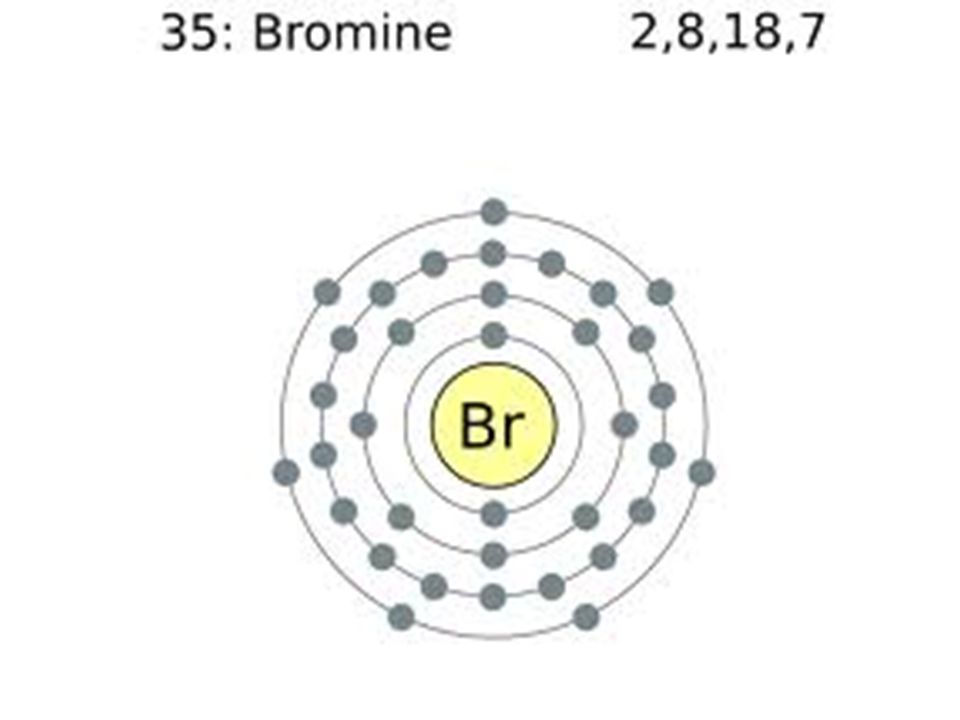
Bromine Electron Configuration Long Form
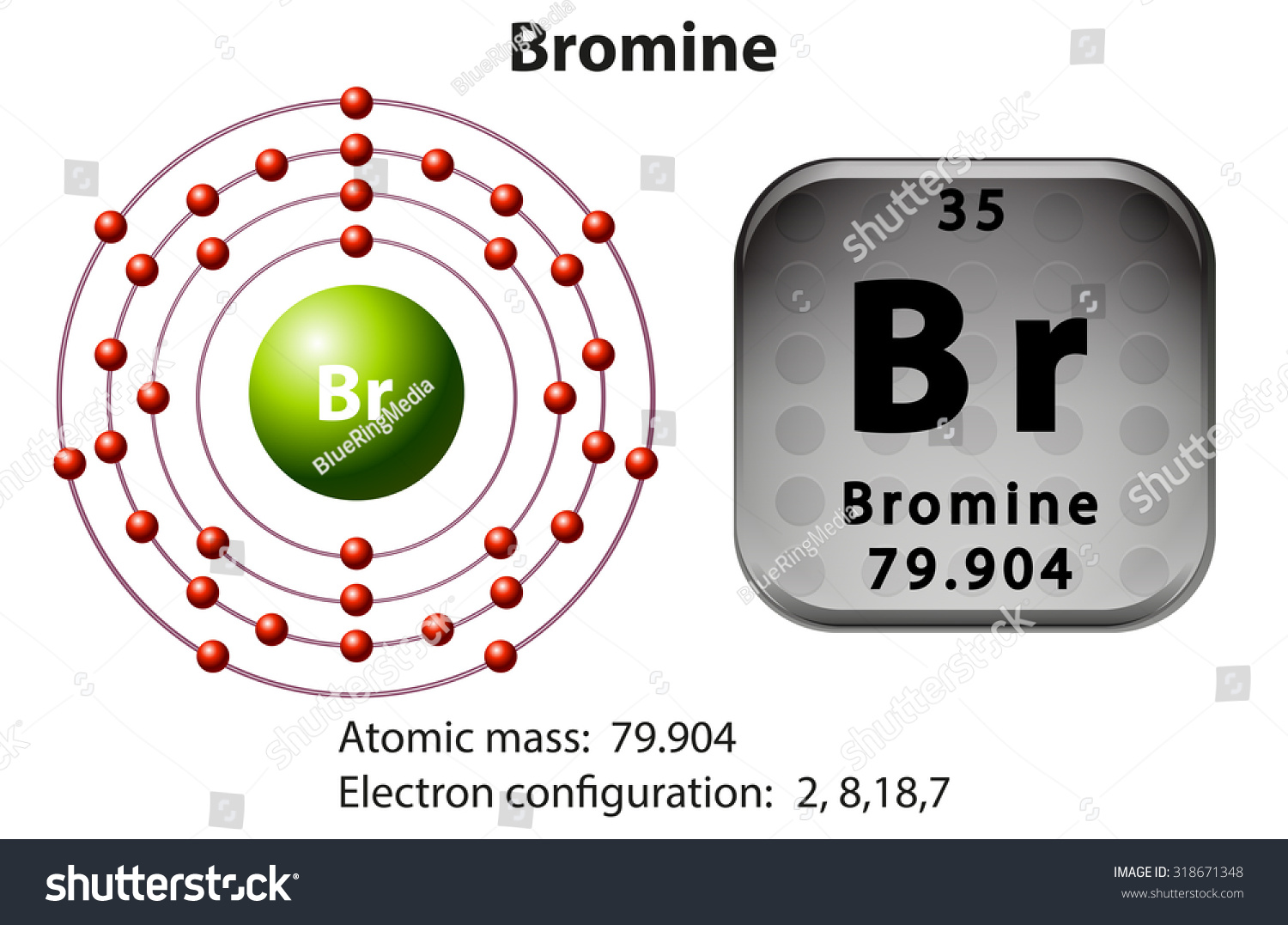
Bromine Electron Configuration Long Form - Web electron configuration of beryllium (be) [he] 2s 2: You may use the core notation or the long form. Typical densities of various substances are at atmospheric pressure. Write the electron configuration for bromine. Some are hard to memorise (or predict), so what is the electron configuration of an atom of br? The atomic number if bromine is 35 thus there are 35 electrons present which fill accorindg to the hund’s rule and aufbau principle a follows: For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. | socratic what is the electron configuration for bromine? Chemistry electron configuration electron configuration 1 answer zach dec 24, 2015 [ar] 4s23d104p5 explanation: Chemical element halogen see all related content → bromine (br), chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 (group viia) of the periodic table.
You can see that 3p is coming before the 4p and after the 4s. Web the arrangement of electrons in an atom by a superscript, in each sublevel is known as electron configuration. Web electron configuration of beryllium (be) [he] 2s 2: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. Density of bromine density of bromine is 3.12g/cm3. Bromine belongs to group 17 which is known as halogens. Web using aufbau principle. Electron configuration of boron (b) [he] 2s 2 2p 1: The orbital filling order is 1s, 2s, 3p, 3s, 4s, 3d, 4p, etc. Science & tech key people:
Electron configuration of oxygen (o. Write the electron configuration for bromine. 1s 2 2s 2 2p 3: So, the valence electronic configuration of group 17 is n s 2 n p 5. What is the complete electron configuration of bromine? Use a chart such as the one below to fill the subshells in order of the diagonal lines. Web the electron configuration of bromine shows that the last shell of bromine has seven electrons. Electronic configuration of group 17: Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. Chemical element halogen see all related content → bromine (br), chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 (group viia) of the periodic table.
Bromine Periodic Table Square Periodic Table Timeline
What is the complete electron configuration of bromine? So, the valence electronic configuration of group 17 is n s 2 n p 5. How many dots should there be in a bromine atom’s lewis symbol? Full electron configuration of bromine: Web electron configuration the arrangements of electrons above the last (closed shell) noble gas.
How Can We Find A Electron Configuration For Bromine (Br)
First, find electrons of bromine atom. Bromine has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5 with the valence electrons in the 4s and 4p orbitals, giving it 7 valence electrons. | socratic what is the electron configuration for bromine? Web there are 35 arrows in the electron.
Bromine ) on emaze
This can be shortened to [ar]4s23d104p5. Full electron configuration of bromine: Electronic configuration of group 17: What is the complete electron configuration of bromine? Chemical element halogen see all related content → bromine (br), chemical element, a deep red noxious liquid, and a member of the halogen elements, or group 17 (group viia) of the periodic table.
Excited State Electron Configuration of Bromine
In lewis electron dot diagram of bromine. Web there are 35 arrows in the electron configuration for bromine, which is for orbital filling. Electronic configuration of group 17: Chemistry electron configuration electron configuration 1 answer zach dec 24, 2015 [ar] 4s23d104p5 explanation: Web total electron pairs exist in the form of lone pairs and bonds.
Bromine Electron Configuration (Br) with Orbital Diagram
You can see that 3p is coming before the 4p and after the 4s. What is the complete electron configuration of bromine? 1s 2 2s 2 2p 3: Web the electron configuration of bromine shows that the last shell of bromine has seven electrons. Density of bromine density of bromine is 3.12g/cm3.
Electron Configuration of Bromine, Br YouTube
These 35 arrows of bromine are only due to the atomic number of bromine. Selenium ← bromine → krypton. Electron configuration of oxygen (o. Science & tech key people: You can see that 3p is coming before the 4p and after the 4s.
Lewis Dot Diagram For Bromine Free Wiring Diagram
Web electron configuration of bromine is [ar] 3d10 4s2 4p5. The elements of group 17 have seven electrons in their outermost shell. Density is defined as the mass per unit volume. Second, make a table of subshell and its maximum electrons. What is the complete electron configuration of bromine?
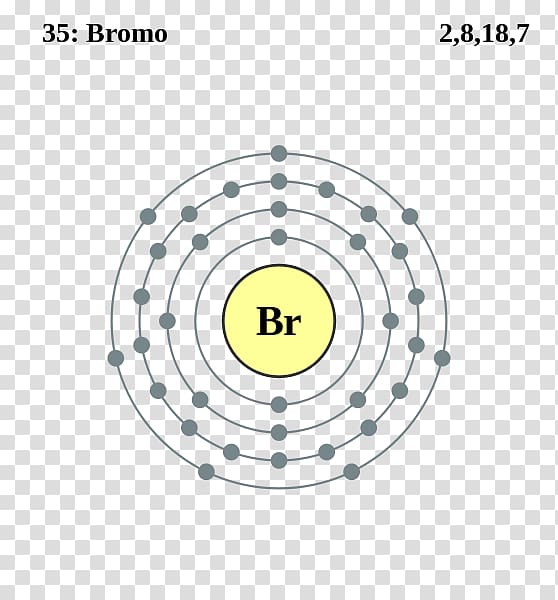
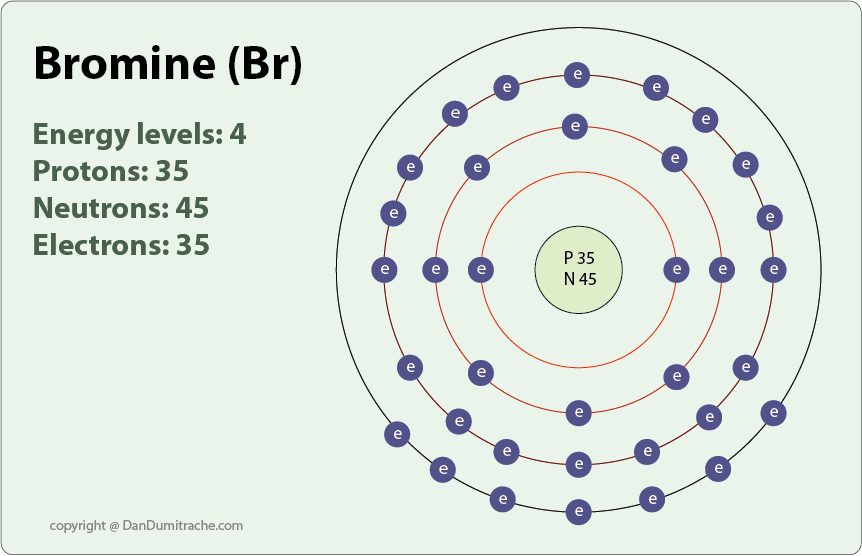
Atom Diagrams Electron Configurations of the Elements
All you need to do is work your way across the periodic table filling the orbitals as you go Electron configuration of boron (b) [he] 2s 2 2p 1: Please be sure to properly use superscripts and subscripts using the t2 or t2 button. This can be shortened to [ar]4s23d104p5. The atomic number if bromine is 35 thus there are.
Orbital Diagram For Rubidium
Density is defined as the mass per unit volume. Bromine has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5 with the valence electrons in the 4s and 4p orbitals, giving it 7 valence electrons. First, find electrons of bromine atom. In lewis electron dot diagram of bromine. Electron.
Bromine Valence Electrons Bromine Valency (Br) Dot Diagram
Write the electron configuration for bromine. So, the valence electronic configuration of group 17 is n s 2 n p 5. Selenium ← bromine → krypton. This can be shortened to [ar]4s23d104p5. How many dots should there be in a bromine atom’s lewis symbol?
Since The Atomic Number Of Bromine Is 35, The Total Electrons Of Bromine Are 35.
What is the complete electron configuration of bromine? 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. The elements of group 17 have seven electrons in their outermost shell. Science & tech key people:
How Many Dots Should There Be In A Bromine Atom’s Lewis Symbol?
Please be sure to properly use superscripts and subscripts using the t2 or t2 button. Bromine has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5 with the valence electrons in the 4s and 4p orbitals, giving it 7 valence electrons. This can be shortened to [ar]4s23d104p5. Melting point (br 2) 265.8 k (−7.2 °c, 19 °f) boiling point (br 2) 332.0 k (58.8 °c, 137.8 °f) density (near r.t.) br 2, liquid:
The Atomic Number If Bromine Is 35 Thus There Are 35 Electrons Present Which Fill Accorindg To The Hund’s Rule And Aufbau Principle A Follows:
Electron configurations of elements beyond hassium (element 108) have never been measured; Web total electron pairs exist in the form of lone pairs and bonds. Therefore, the valence electrons of bromine are seven. 1s 2 2s 2 2p 2:
Web Let’s Use It To Write The Electron Configuration Of A Neutral Bromine Atom, A Bromine Atom Has 35 Electrons.
You may use the core notation or the long form. Web feb 28, 2018 the electron configuration of bromine is 1s22s22p63s23p64s23d104p5. The complete electron configuration of bromine is 1s22s2 2p63s23p64s2 3d10 4p5. Using the blocks in the periodic table we can write the electron configuration of bromine as:


:max_bytes(150000):strip_icc()/Bromine-58b601f93df78cdcd83d2817.jpg)