Can Nh3 Form Hydrogen Bonds
Can Nh3 Form Hydrogen Bonds - Hence, ph 3 cannot form hydrogen bonds. Ph 3 exhibits a trigonal pyramidal molecular geometry like that of ammonia, but unlike nh 3 it cannot hydrogen bond. Hydrogen bonding is exhibited in hf,. Note that the o atom in one molecule is attracted to a h atom in the second molecule. To understand hydrogen bonding in ammonia (nh3) we need to know that ammonia is a polar molecule. Web only ch₃nh₂ and ch₃oh can have hydrogen bonds between other molecules of the same kind. 8.9k views 1 year ago. Web any given nh_3 molecule can form 4 hydrogen bonds, but on average they can only participate in two hydrogen bonds, o. Nh3 can form hydrogen bonds. Note that the n atom in the nh 3 molecule is attracted to a h atom in the h 2 o molecule.
But, in bulk nh[math]_3[/math], you soon have too many hydrogens and not enough electron pairs. However, hf is a weak acid, h2o is amphoteric, and nh3 is a weak base. P has large size and low electronegativity. To have hydrogen bonding, you need an n, o, or f atom in one molecule and an h attached to an n, o, or f atom in another molecule. The attractions are still there and always will be, but the molecules collide with enough force to overcome the attractions. Web hydrogen bonding between two water (h 2 o) molecules. Web only ch₃nh₂ and ch₃oh can have hydrogen bonds between other molecules of the same kind. In the ammonia molecule group, the lone pair of electrons is not enough for forming the hydrogen bond. Web any given nh_3 molecule can form 4 hydrogen bonds, but on average they can only participate in two hydrogen bonds, o. Note that the o atom in one molecule is attracted to a h atom in the second molecule.
In water, hcl, hbr, and hi all act as strong acids just as hno3 does. Nh3 can form hydrogen bonds. This means that it has a positive and a negative side. Web answer (1 of 3): Hydrogen bonding is the intermolecular forces acting between ammonia molecules. The attractions are still there and always will be, but the molecules collide with enough force to overcome the attractions. Web solution verified by toppr n has small atomic size and high electronegativity. No, nitric acid is a strong acid and exists, in aqueous solution, as separated hydrogen ions and nitrate ions. $\ce{nh3}$ why isn't $\ce{ch3och3}$ since $\ce{h}$ is bonded with $\ce{o}$ whereas $\ce{h}$ in $\ce{nh3}$ is not bonded to any oxygens? Surprisingly, no evidence has been found to support the view that nh 3 acts as.
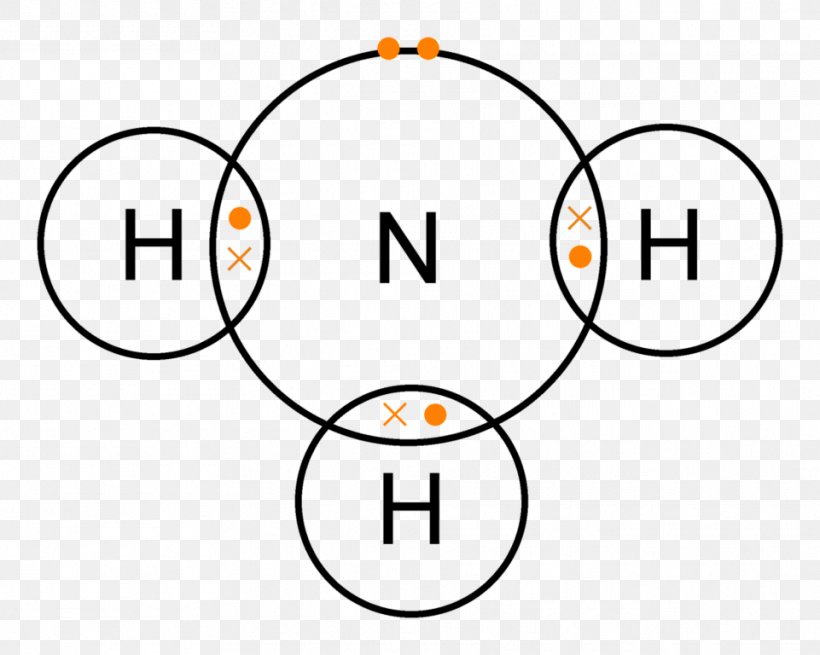
Lewis Structure Ammonia Covalent Bond Lone Pair Chemical Bond, PNG
To understand hydrogen bonding in ammonia (nh3) we need to know that ammonia is a polar molecule. Surprisingly, no evidence has been found to support the view that nh 3 acts as. Web answer (1 of 3): 8.9k views 1 year ago. Hydrogen bonding is exhibited in hf,.
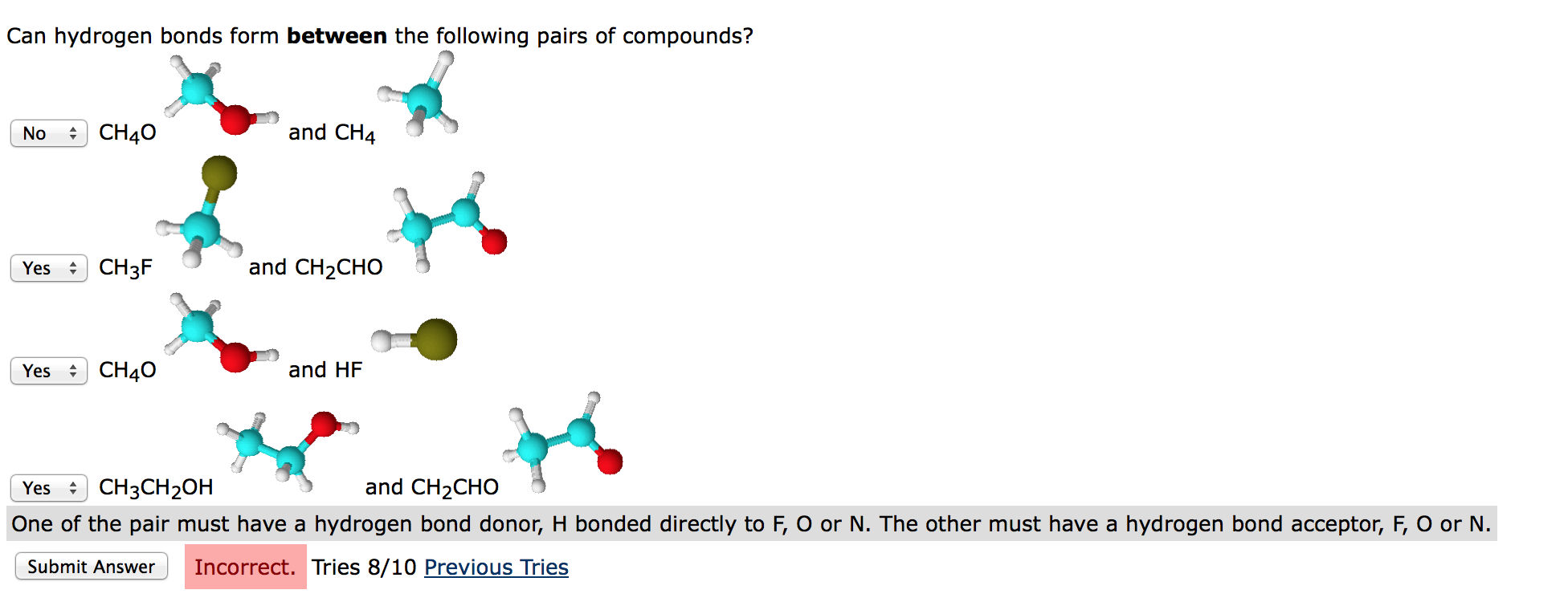
Solved Can hydrogen bonds form between the following pairs
Hydrogen bonding between a water molecule and an ammonia (nh 3) molecule. Hence, nh 3 can form hydrogen bonds. Web answer (1 of 3): So yes, we can have hydrogen bonding between one h2o molecule and one hcl molecule, in which case the o molecule in h2o forms a hydrogen bond with the h from hcl. Web to compare (nh.
Solved Part A Which of the following molecules can form
\[ nh_3 + h_2o \rightleftharpoons nh_4^+. Hence, ph 3 cannot form hydrogen bonds. Thus hydrogen bonding can account for the unusually high boiling points of nh 3, h 2 o, and hf. Hence, nh 3 can form hydrogen bonds. This is because hydrogen bonds can be formed when hydrogen is covalently bonded to a highly electronegative atom like.
Bonds That Hold Water Molecules Together / Intermolecular Forces
\[ nh_3 + h_2o \rightleftharpoons nh_4^+. Hence, ph 3 cannot form hydrogen bonds. This means that it has a positive and a negative side. Web solution verified by toppr n has small atomic size and high electronegativity. Web ammonia has the ability to form hydrogen bonds.
Solved Which of the following molecules can form hydrogen
Web yes, nh3 forms hydrogen bonds. P has large size and low electronegativity. However, hf is a weak acid, h2o is amphoteric, and nh3 is a weak base. Note that the n atom in the nh 3 molecule is attracted to a h atom in the h 2 o molecule. Ph 3 exhibits a trigonal pyramidal molecular geometry like that.
Does NH3 have Hydrogen Bonding Techiescientist
Web hydrogen bonding between two water (h 2 o) molecules. Hence, ph 3 cannot form hydrogen bonds. The attractions are still there and always will be, but the molecules collide with enough force to overcome the attractions. No, nitric acid is a strong acid and exists, in aqueous solution, as separated hydrogen ions and nitrate ions. In the ammonia molecule.
Part A Which of the following molecules can form hydrogen bonds? NaH
Hence, nh 3 can form hydrogen bonds. $\ce{nh3}$ why isn't $\ce{ch3och3}$ since $\ce{h}$ is bonded with $\ce{o}$ whereas $\ce{h}$ in $\ce{nh3}$ is not bonded to any oxygens? Hydrogen bonding is exhibited in hf,. Nh3 can form hydrogen bonds. In the gaseous state at high temperature, the ammonia molecules have sufficient kinetic energy to overcome the attractions to other ammonia molecules.
chemistry Intermolecular Hydrogen Bonding
Web to compare (nh 3) 2, we calculated the energy density difference of (nh 3) 2 (see figure s7), and there are more charge depletions in h of the hydrogen bond relative to free h, indicating that h atoms of the hydrogen bond. Thus, we see molecules such as ph 3, which do not participate in hydrogen bonding. In the.
How do Hydrogen bonds form in H2O NH3 HF Hydrogen Bonding
Hence, nh 3 can form hydrogen bonds. Web solution verified by toppr n has small atomic size and high electronegativity. To have hydrogen bonding, you need an n, o, or f atom in one molecule and an h attached to an n, o, or f atom in another molecule. Web which of the following compounds can form hydrogen bonds? $\ce{nh3}$.
How many hydrogen bonds form by Nh3, H20 and HF and boiling point trend
This means that it has a positive and a negative side. Ammonia has electronegative atom nitrogen connected to hydrogen atoms. 8.9k views 1 year ago. \[ nh_3 + h_2o \rightleftharpoons nh_4^+. Web which of the following compounds can form hydrogen bonds?
Note That The N Atom In The Nh 3 Molecule Is Attracted To A H Atom In The H 2 O Molecule.
$\ce{nh3}$ why isn't $\ce{ch3och3}$ since $\ce{h}$ is bonded with $\ce{o}$ whereas $\ce{h}$ in $\ce{nh3}$ is not bonded to any oxygens? Note that the o atom in one molecule is attracted to a h atom in the second molecule. P has large size and low electronegativity. To understand hydrogen bonding in ammonia (nh3) we need to know that ammonia is a polar molecule.
Nh3 Can Form Hydrogen Bonds.
Thus, we see molecules such as ph 3, which do not participate in hydrogen bonding. This is because hydrogen bonds can be formed when hydrogen is covalently bonded to a highly electronegative atom like. It can, for a single molecule of ammonia surrounded by other suitable molecules. However, hf is a weak acid, h2o is amphoteric, and nh3 is a weak base.
Thus Hydrogen Bonding Can Account For The Unusually High Boiling Points Of Nh 3, H 2 O, And Hf.
Web only ch₃nh₂ and ch₃oh can have hydrogen bonds between other molecules of the same kind. Web any given nh_3 molecule can form 4 hydrogen bonds, but on average they can only participate in two hydrogen bonds, o. When the hydrogen bonds between water molecules are broken, they can be replaced by equivalent bonds between water and ammonia molecules. Web answer (1 of 3):
To Have Hydrogen Bonding, You Need An N, O, Or F Atom In One Molecule And An H Attached To An N, O, Or F Atom In Another Molecule.
Hence, ph 3 cannot form hydrogen bonds. Hence, nh 3 can form hydrogen bonds. Web hydrogen bonding cannot occur without significant electronegativity differences between hydrogen and the atom it is bonded to. Due to the electronegativity difference between the nitrogen atom and hydrogen, a partial negative charge develops on nitrogen while a partial positive charge develops on the hydrogen atom.