Chemical Bonds Are Likely To Form When
Chemical Bonds Are Likely To Form When - Sometimes, it is enough to bring two substances together for a chemical reaction to begin, but often an external stimulus. Chemical bonds are likely to form when _____. The bonded atoms may be of the same element, as in the case of h 2, which is called molecular hydrogen or hydrogen gas. Web when the difference is very small or zero, the bond is covalent and nonpolar. Web one way to predict the type of bond that forms between two elements is to compare the electronegativities of the elements. In general, large differences in electronegativity result in ionic bonds, while smaller differences result in covalent bonds. When it is large, the bond is polar covalent or ionic. Web answer (1 of 18): Web chemical bonds are forces that hold the atoms together in a molecule. Web chemical bonds are likely to form when a.
Web chemical bonds are forces that hold the atoms together in a molecule. Web all members of a particular group have analogous outermost (valence) electron configurations, suggesting that all members of a group should show a family relationship in the types and numbers of the chemical bonds that they are able to form. Some atoms become more stable by gaining or losing an entire electron (or several electrons). Web if atoms have similar electronegativities (the same affinity for electrons), covalent bonds are most likely to occur. In general, large differences in electronegativity result in ionic bonds, while smaller differences result in covalent bonds. B) an atom’s outer energy level is filled to capacity. Web a more or less stable grouping of two or more atoms held together by chemical bonds is called a molecule. A) an atom’s outer energy level doesn’t have the maximum number of electrons. Sometimes, it is enough to bring two substances together for a chemical reaction to begin, but often an external stimulus. Chemical bonds are likely to form when _____.
Web all members of a particular group have analogous outermost (valence) electron configurations, suggesting that all members of a group should show a family relationship in the types and numbers of the chemical bonds that they are able to form. The bonded atoms may be of the same element, as in the case of h 2, which is called molecular hydrogen or hydrogen gas. Because both atoms have the same affinity for electrons and neither has a tendency to donate them, they share electrons in order to. When two atoms approach each. Sometimes, it is enough to bring two substances together for a chemical reaction to begin, but often an external stimulus. A) an atom’s outer energy level doesn’t have the maximum number of electrons. Web chemical bonds are forces that hold the atoms together in a molecule. When they do so, atoms form ions, or charged particles. B) an atom’s outer energy level is filled to capacity. They are a result of strong intramolecular interactions among the atoms of a molecule.
PPT Mastering Chemistry PowerPoint Presentation, free download ID
The bonded atoms may be of the same element, as in the case of h 2, which is called molecular hydrogen or hydrogen gas. Web a more or less stable grouping of two or more atoms held together by chemical bonds is called a molecule. Because both atoms have the same affinity for electrons and neither has a tendency to.
CHEMICAL BONDING CHEMICAL BONDING
B) an atom’s outer energy level is filled to capacity. Bond length and bond energy (opens a modal) worked example: Web one way to predict the type of bond that forms between two elements is to compare the electronegativities of the elements. An atom’s nucleus has the same number of protons as it does neutrons two atoms have the same.
What Are Chemical Bonds and Why Do They Form?
Web a chemical bond is formed to stabilize the outermost shell in an element. Interpreting potential energy curves of diatomic molecules (opens a modal) lattice energy (opens a modal) ionic bonds and. Web chemical bonds are likely to form when a. Bond length and bond energy (opens a modal) worked example: Web one way to predict the type of bond.
Three Chemical Bonds Romance Abounds! Brain BREAK!
A) an atom’s outer energy level doesn’t have the maximum number of electrons. Sometimes, it is enough to bring two substances together for a chemical reaction to begin, but often an external stimulus. Web one way to predict the type of bond that forms between two elements is to compare the electronegativities of the elements. When a molecule is made.
17 Best images about Science on Pinterest Dna, Funny science and Student
Chemical bonds are likely to form when _____. Web if atoms have similar electronegativities (the same affinity for electrons), covalent bonds are most likely to occur. B) or the number of electrons are lesser than the. Because both atoms have the same affinity for electrons and neither has a tendency to donate them, they share electrons in order to. C).
Types of Chemical Bonds WIRDOU
Web a more or less stable grouping of two or more atoms held together by chemical bonds is called a molecule. Interpreting potential energy curves of diatomic molecules (opens a modal) lattice energy (opens a modal) ionic bonds and. C) an atom’s nucleus has the same number of. Two atoms have the same number of electrons. Web three types of.
PPT Lecture 21 Chemical Bonding PowerPoint Presentation, free
Web if atoms have similar electronegativities (the same affinity for electrons), covalent bonds are most likely to occur. Web a chemical bond is formed to stabilize the outermost shell in an element. The bonded atoms may be of the same element, as in the case of h 2, which is called molecular hydrogen or hydrogen gas. B) an atom’s outer.
Why Do Most Atoms Form Chemical Bonds? Sciencing
B) an atom’s outer energy level is filled to capacity. Web chemical bonds are likely to form when a. Web answer (1 of 18): Bond length and bond energy (opens a modal) worked example: Web all members of a particular group have analogous outermost (valence) electron configurations, suggesting that all members of a group should show a family relationship in.
Bonds Definition and Examples in Chemistry
Interpreting potential energy curves of diatomic molecules (opens a modal) lattice energy (opens a modal) ionic bonds and. Electron gain or loss can give an atom a filled outermost. When it is large, the bond is polar covalent or ionic. The bonded atoms may be of the same element, as in the case of h 2, which is called molecular.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
When two atoms approach each. In general, large differences in electronegativity result in ionic bonds, while smaller differences result in covalent bonds. Sometimes, it is enough to bring two substances together for a chemical reaction to begin, but often an external stimulus. Some atoms become more stable by gaining or losing an entire electron (or several electrons). Web chemical bonds.
In General, Large Differences In Electronegativity Result In Ionic Bonds, While Smaller Differences Result In Covalent Bonds.
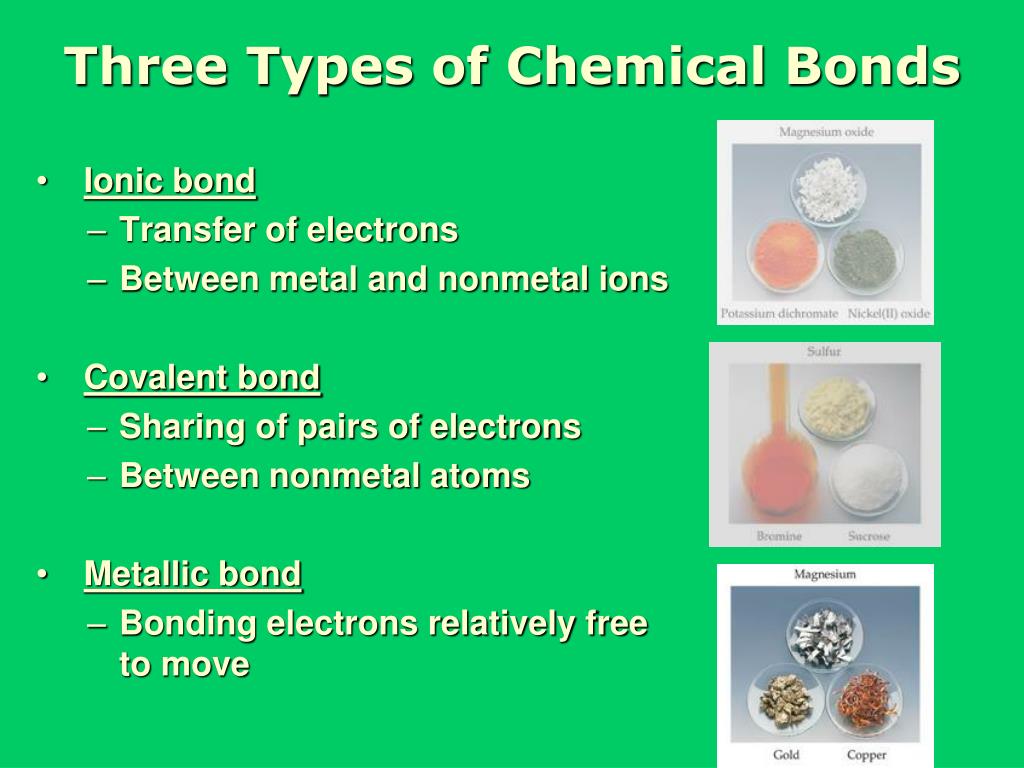
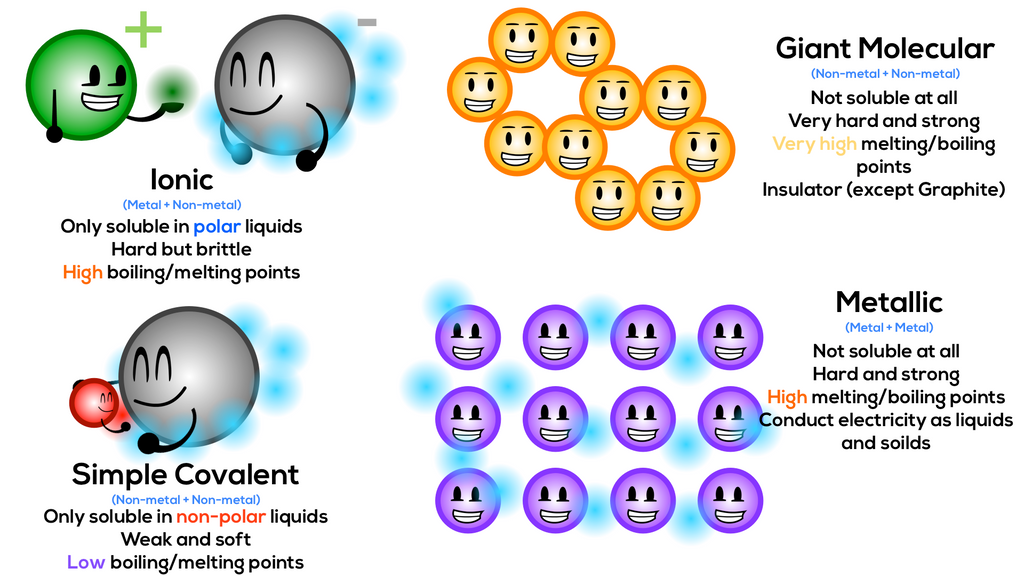
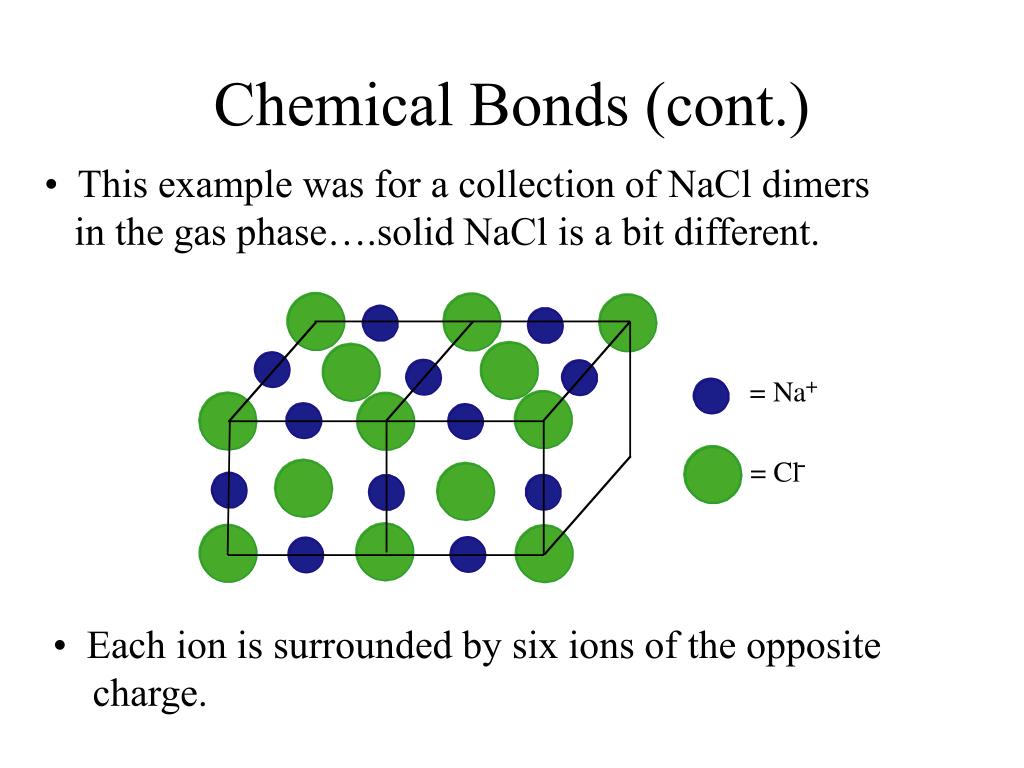
B) an atom’s outer energy level is filled to capacity. Web three types of chemical bonds are important in human physiology, because they hold together substances that are used by the body for critical aspects of homeostasis, signaling, and energy production, to name just a few important processes. Some atoms become more stable by gaining or losing an entire electron (or several electrons). Two atoms have the same number of electrons.
When Two Atoms Approach Each.
Web chemical bonds are forces that hold the atoms together in a molecule. Web a chemical bond is formed to stabilize the outermost shell in an element. Web chemical bonds are likely to form when a. Web all members of a particular group have analogous outermost (valence) electron configurations, suggesting that all members of a group should show a family relationship in the types and numbers of the chemical bonds that they are able to form.
For A Chemical Bond To Form, The Elements Must Have The Last Level Of Energy That Is Not Complete, To Be Able To Complete It Through Chemical Bonding.
They are a result of strong intramolecular interactions among the atoms of a molecule. Web these bonds form when an electron is shared between two elements and are the strongest and most common form of chemical bond in living organisms. Web types of chemical bonds. Bond length and bond energy (opens a modal) worked example:
The Atoms In Group 6A Make Two Covalent Bonds.
Chemical bond formation takes place between two or more atoms.the forces of attraction between the nucleus of one atom and the electrons of the other and the forces of repulsion between the nucleus of two atoms and the forces of repulsion. Web during these chemical reactions, the original molecules break apart and form new bonds to produce different materials. C) an atom’s nucleus has the same number of. Interpreting potential energy curves of diatomic molecules (opens a modal) lattice energy (opens a modal) ionic bonds and.





/GettyImages-724235099-5a85a8c4119fa80037c0cb00.jpg)
