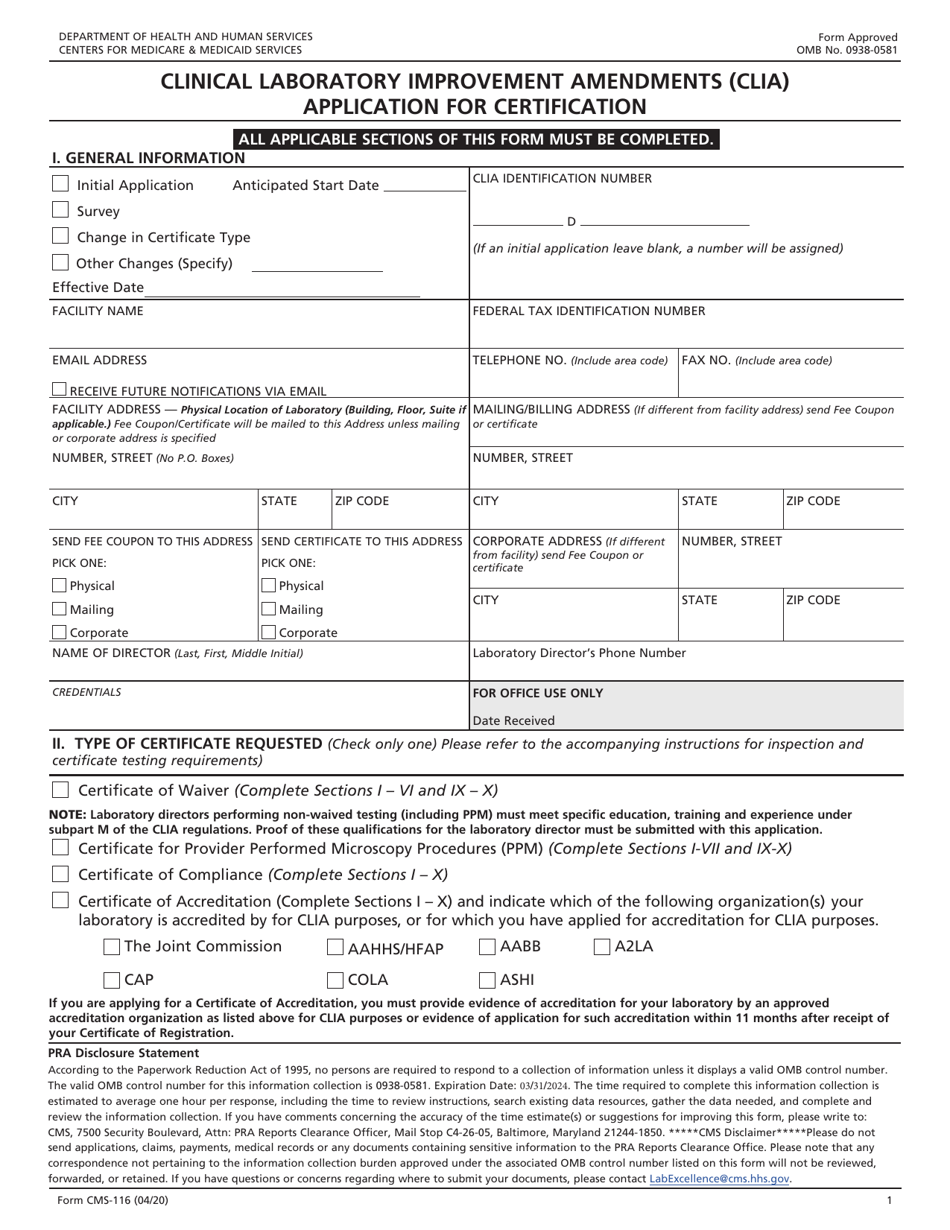
Clia Application Form
Clia Application Form - This guide helps laboratories seeking to apply for clia certification from cms. Web clia applications and certificate updates. Clinical laboratory improvement amendments of 1988 (clia) application for certification. Web how do i apply for a clia certificate? Sas (pdf) process clia applications (pdf), renewals, updates, and requests for certificate copies. More information can be found on the cms clia website. Web applying for a clia certificate what form do i use to apply? Web search a database of documents related to clia from these sources: Clia has regulatory requirements for quality that all laboratories must meet. Although clia is a federal program, state agencies (sas) are responsible for laboratory oversight and maintaining clia laboratories’ certification records.
Clinical laboratory improvement amendments of 1988 (clia) waiver applications for manufacturers of in vitro diagnostic devices. This guide helps laboratories seeking to apply for clia certification from cms. Send your completed application to the address of the local state agency for the state in which your laboratory is located. Division of laboratory systems (dls) about clia. Web clia applications and certificate updates. Contact health facility compliance at infohflc@hhs.texas.gov with any questions. Clinical laboratory improvement amendments (clia) application for certification all applicable sections of this form must be completed. Send your completed application to the address of the local state agency for the state in which your laboratory is located. Sas (pdf) process clia applications (pdf), renewals, updates, and requests for certificate copies. More information can be found on the cms clia website.
Sas (pdf) process clia applications (pdf), renewals, updates, and requests for certificate copies. Web applying for a clia certificate what form do i use to apply? Send your completed application to the address of the local state agency for the state in which your laboratory is located. Additionally, check with your state agency for any other The code of federal regulations. Although clia is a federal program, state agencies (sas) are responsible for laboratory oversight and maintaining clia laboratories’ certification records. Clinical laboratory improvement amendments of 1988 (clia) waiver applications for manufacturers of in vitro diagnostic devices. This guide helps laboratories seeking to apply for clia certification from cms. Division of laboratory systems (dls) about clia. Send your completed application to the address of the local state agency for the state in which your laboratory is located.
Clia Application Form 0938 0581 20202022 Fill and Sign Printable
More information can be found on the cms clia website. Clinical laboratory improvement amendments of 1988 (clia) application for certification. Web how do i apply for a clia certificate? Clinical laboratory improvement amendments of 1988 (clia) waiver applications for manufacturers of in vitro diagnostic devices. Web clia applications and certificate updates.
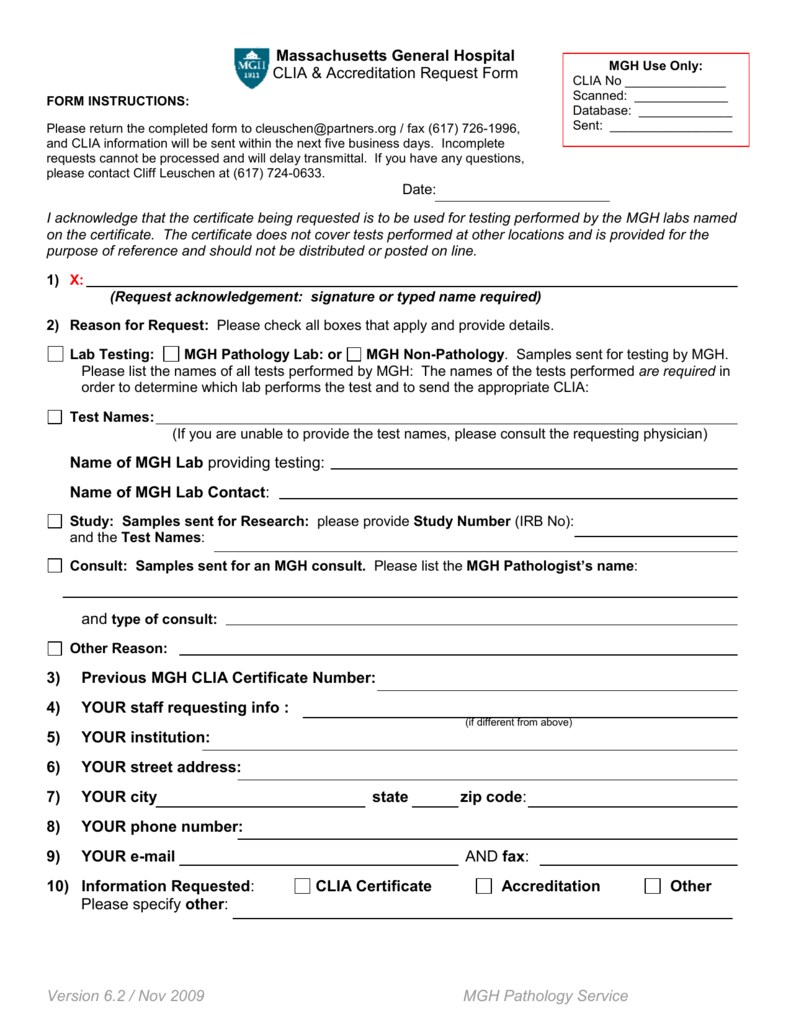
CLIA Request Form Massachusetts General Hospital
Clinical laboratory improvement amendments of 1988 (clia) application for certification. Although clia is a federal program, state agencies (sas) are responsible for laboratory oversight and maintaining clia laboratories’ certification records. Web search a database of documents related to clia from these sources: Send your completed application to the address of the local state agency for the state in which your.
Illinois Clia Certificate Type Change Form Download Fillable PDF
Web clia applications and certificate updates. Send your completed application to the address of the local state agency for the state in which your laboratory is located. Web search a database of documents related to clia from these sources: Division of laboratory systems (dls) about clia. Send your completed application to the address of the local state agency for the.
Certification Programs CLIA
The code of federal regulations. Contact health facility compliance at infohflc@hhs.texas.gov with any questions. This guide helps laboratories seeking to apply for clia certification from cms. Consider submitting planned protocols or study. Clia has regulatory requirements for quality that all laboratories must meet.
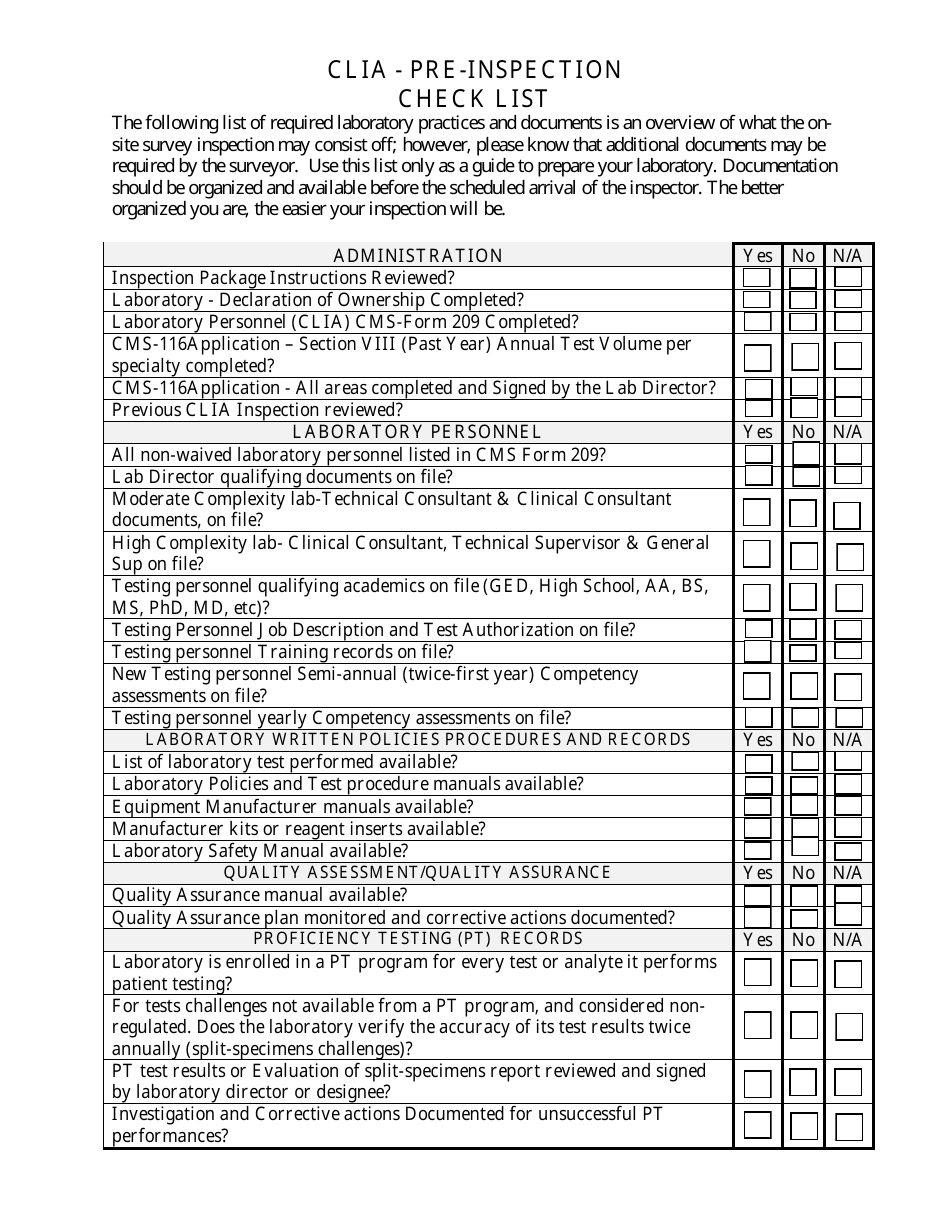
Illinois Clia Preinspection Check List Download Fillable PDF
Web clia applications and certificate updates. Consider submitting planned protocols or study. More information can be found on the cms clia website. Send your completed application to the address of the local state agency for the state in which your laboratory is located. This guide helps laboratories seeking to apply for clia certification from cms.
How to Apply for a CLIA Certificate? Filling out CMS116 Lighthouse
Clinical laboratory improvement amendments of 1988 (clia) waiver applications for manufacturers of in vitro diagnostic devices. Although clia is a federal program, state agencies (sas) are responsible for laboratory oversight and maintaining clia laboratories’ certification records. Clia has regulatory requirements for quality that all laboratories must meet. Division of laboratory systems (dls) about clia. Web applying for a clia certificate.
Clia Application Cms 116 Form ≡ Fill Out Printable PDF Forms Online
Clinical laboratory improvement amendments of 1988 (clia) application for certification. Additionally, check with your state agency for any other Web applying for a clia certificate what form do i use to apply? Although clia is a federal program, state agencies (sas) are responsible for laboratory oversight and maintaining clia laboratories’ certification records. Send your completed application to the address of.
Form CMS116 Download Fillable PDF or Fill Online Clinical Laboratory
Clinical laboratory improvement amendments of 1988 (clia) waiver applications for manufacturers of in vitro diagnostic devices. Web how do i apply for a clia certificate? More information can be found on the cms clia website. Sas (pdf) process clia applications (pdf), renewals, updates, and requests for certificate copies. Web applying for a clia certificate what form do i use to.
Form CL3 Download Printable PDF or Fill Online Application for a
Clinical laboratory improvement amendments of 1988 (clia) application for certification. Although clia is a federal program, state agencies (sas) are responsible for laboratory oversight and maintaining clia laboratories’ certification records. The code of federal regulations. Code of federal regulations (cfr): Web search a database of documents related to clia from these sources:
Form CMS116 Download Fillable PDF, Clinical Laboratory Improvement
Web search a database of documents related to clia from these sources: Send your completed application to the address of the local state agency for the state in which your laboratory is located. Consider submitting planned protocols or study. Web clia applications and certificate updates. Send your completed application to the address of the local state agency for the state.
Web Search A Database Of Documents Related To Clia From These Sources:
Clinical laboratory improvement amendments of 1988 (clia) waiver applications for manufacturers of in vitro diagnostic devices. Clinical laboratory improvement amendments of 1988 (clia) application for certification. Although clia is a federal program, state agencies (sas) are responsible for laboratory oversight and maintaining clia laboratories’ certification records. Additionally, check with your state agency for any other
This Guide Helps Laboratories Seeking To Apply For Clia Certification From Cms.
Division of laboratory systems (dls) about clia. Send your completed application to the address of the local state agency for the state in which your laboratory is located. More information can be found on the cms clia website. Web applying for a clia certificate what form do i use to apply?
Code Of Federal Regulations (Cfr):
Web clia applications and certificate updates. Web how do i apply for a clia certificate? Sas (pdf) process clia applications (pdf), renewals, updates, and requests for certificate copies. Consider submitting planned protocols or study.
Contact Health Facility Compliance At Infohflc@Hhs.texas.gov With Any Questions.
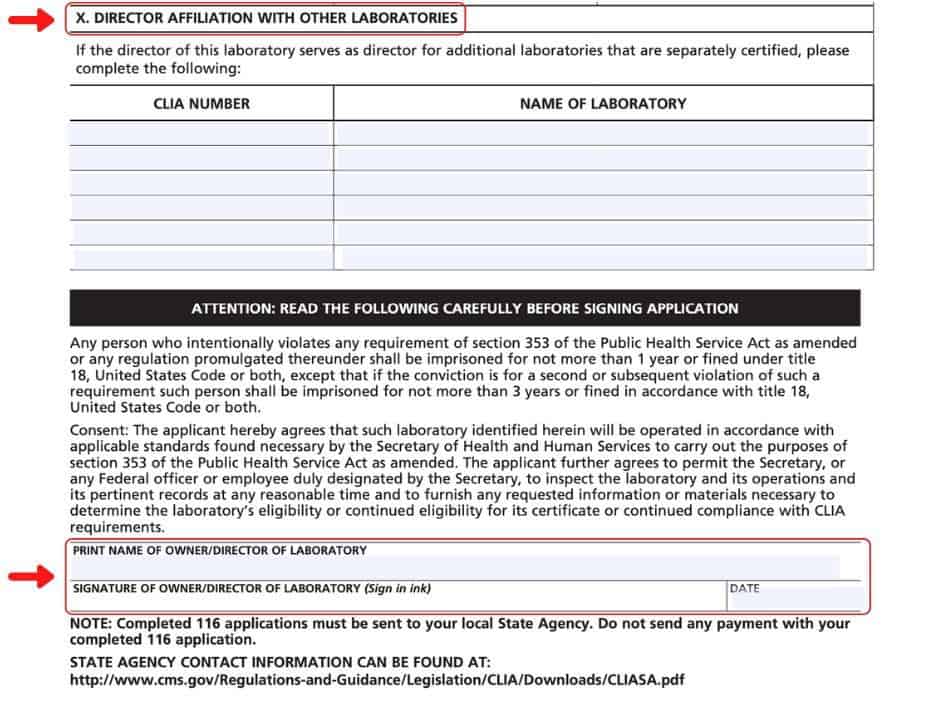
The code of federal regulations. Clia has regulatory requirements for quality that all laboratories must meet. Send your completed application to the address of the local state agency for the state in which your laboratory is located. Clinical laboratory improvement amendments (clia) application for certification all applicable sections of this form must be completed.