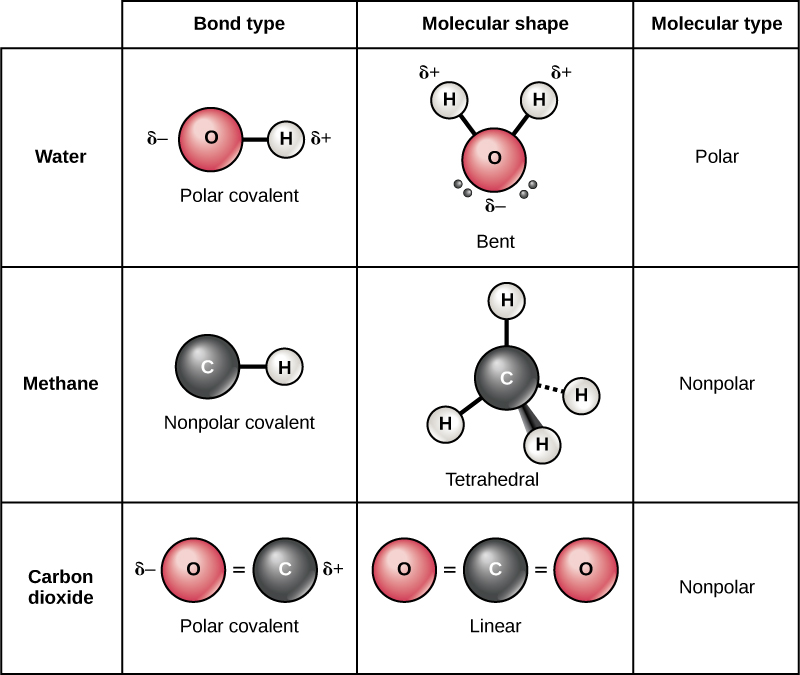
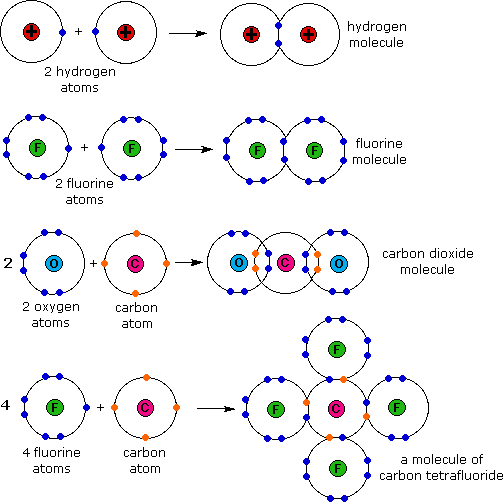
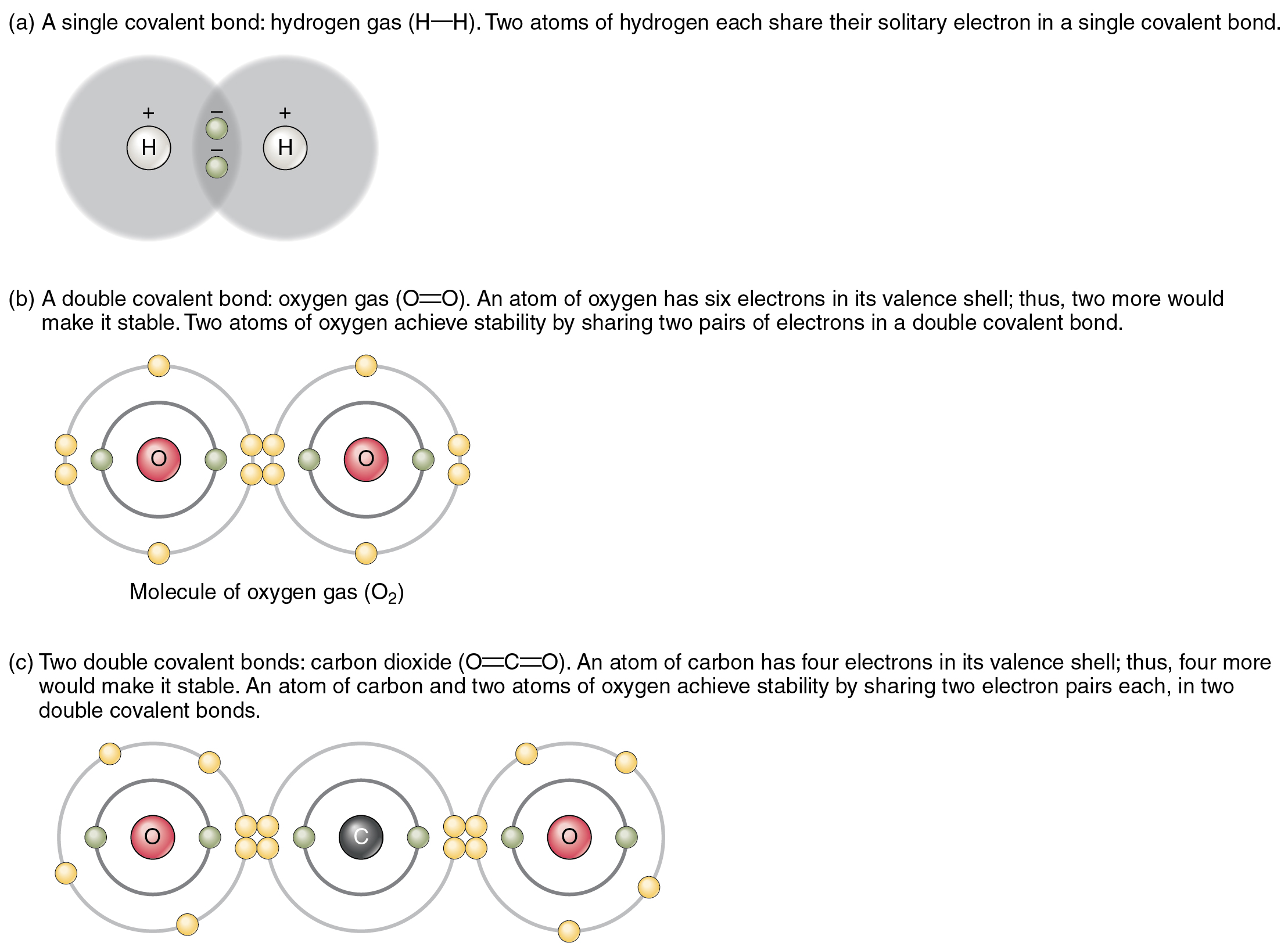
How Many Covalent Bonds Can Oxygen Form
How Many Covalent Bonds Can Oxygen Form - Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. To fill its valence shell, oxygen needs two additional electrons, and hydrogen needs one. Those are the outermost electrons,. Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. Atoms of different elements will form either one,. Web can oxygen have 4 covalent bonds? Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. This is false that oxygen atom can form four covalent bonds with up to four other atoms. However, if you give it a positive charge by removing an electron, then it would only have 5 5 electrons in its. Fluorine and the other halogens in group 7a (17) have seven valence electrons and can.
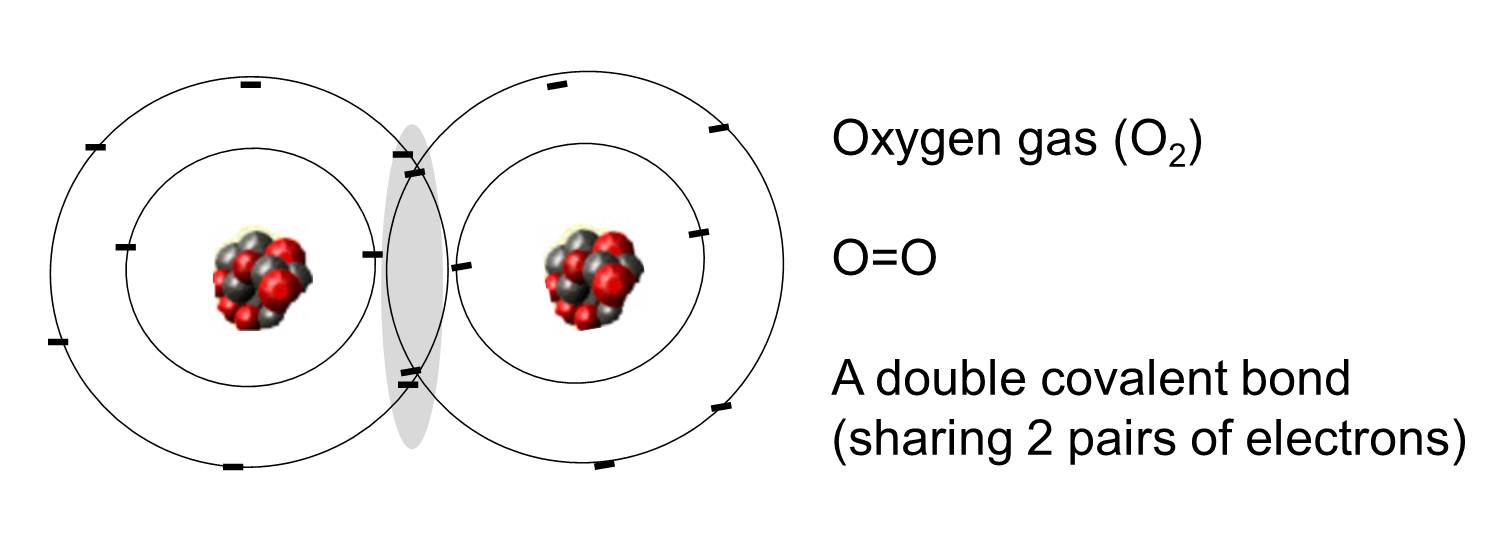
Therefore, it has six valence electrons. Web in order to form a covalent bond, each element has to share one unpaired electron. The number of covalent bonds that an atom can form is dictated by how many valence electrons it has. Web test 1 2 3 4 5 covalent bonds forming a covalent bond a covalent bond is formed when two atoms share a pair of electrons. Web therefore, oxygen can form 2 single covalent bonds. Fluorine and the other halogens in group 7a (17) have seven valence. Web can oxygen have 4 covalent bonds? This is false that oxygen atom can form four covalent bonds with up to four other atoms. Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. Those are the outermost electrons,.
Don’t get confused as in this question single oxygen atom (o) is asked not for dioxygen o2 o 2. To fill its valence shell, oxygen needs two additional electrons, and hydrogen needs one. Web can oxygen have 4 covalent bonds? Oxygen can form two single bonds because it has six valent electrons on its outer shell. Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. Web test 1 2 3 4 5 covalent bonds forming a covalent bond a covalent bond is formed when two atoms share a pair of electrons. In general, the number and type of bonds possible with any element are determined by the valence electrons. It is easier for an oxygen atom to accept or share two. Web in order to form a covalent bond, each element has to share one unpaired electron. Web oxygen has 6 6 electrons in its outermost shell.
Liquid Properties Boundless Chemistry
Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Fluorine and the other halogens in group 7a (17) have seven valence. Those are the outermost electrons,. It is easier for an oxygen atom to accept or share two. Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds.
5.2 Bonding and Lattices Physical Geology, First University of
In general, the number and type of bonds possible with any element are determined by the valence electrons. However, if you give it a positive charge by removing an electron, then it would only have 5 5 electrons in its. It is easier for an oxygen atom to accept or share two. Nonmetal atoms frequently form covalent bonds with other.
Covalent bonding in an oxygen molecule.
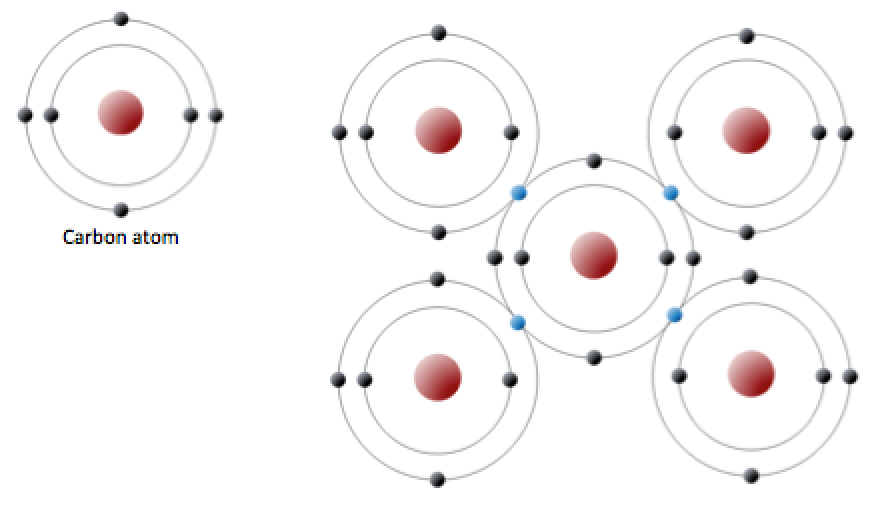
Web therefore, oxygen can form 2 single covalent bonds. Web carbon will form four covalent bonds, nitrogen will form three covalent bonds, oxygen will form two covalent bonds, and hydrogen will form one covalent. Fluorine and the other halogens in group 7a (17) have seven valence. Web can oxygen have 4 covalent bonds? Atoms of different elements will form either.
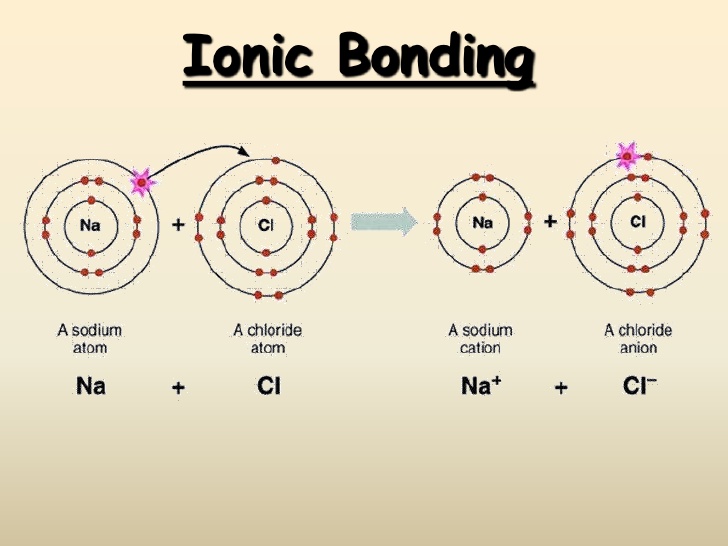
What are similarities of covalent and ionic bonding? Socratic
Fluorine and the other halogens in group 7a (17) have seven valence. Web carbon will form four covalent bonds, nitrogen will form three covalent bonds, oxygen will form two covalent bonds, and hydrogen will form one covalent. Web therefore, oxygen can form 2 single covalent bonds. Web carbon will form four covalent bonds, nitrogen will form three covalent bonds, oxygen.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Web therefore, oxygen can form 2 single covalent bonds. However, if you give it a positive charge by removing an electron, then it would only have 5 5 electrons in its. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Web in order to form a covalent bond, each element has to share one unpaired electron. Web a covalent.
Basic Cell Biology
Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Web formation of covalent bonds. Web carbon will form four covalent bonds, nitrogen will form three covalent bonds, oxygen will form two covalent bonds, and hydrogen will form one covalent. Fluorine and the other halogens in group 7a (17) have seven valence electrons and can. Oxygen can form two single.
Atoms, Isotopes, Ions, and Molecules The Building Blocks · Biology
Web therefore, oxygen can form 2 single covalent bonds. Web carbon will form four covalent bonds, nitrogen will form three covalent bonds, oxygen will form two covalent bonds, and hydrogen will form one covalent. Web a covalent bond is formed by two shared electrons. Web can oxygen have 4 covalent bonds? Web oxygen has 6 6 electrons in its outermost.
What's the difference between a formula unit and a molecule? Socratic
Web in order to form a covalent bond, each element has to share one unpaired electron. This is false that oxygen atom can form four covalent bonds with up to four other atoms. Atoms of different elements will form either one,. Web therefore, oxygen can form 2 single covalent bonds. Therefore, it has six valence electrons.
Online Essay Help amazonia.fiocruz.br
In general, the number and type of bonds possible with any element are determined by the valence electrons. Web oxygen has 6 6 electrons in its outermost shell. Web test 1 2 3 4 5 covalent bonds forming a covalent bond a covalent bond is formed when two atoms share a pair of electrons. Web formation of covalent bonds. It.
Chemical Bonds Anatomy & Physiology
Web therefore, oxygen can form 2 single covalent bonds. This is false that oxygen atom can form four covalent bonds with up to four other atoms. The number of covalent bonds that an atom can form is dictated by how many valence electrons it has. Those are the outermost electrons,. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms.
Web Oxygen Has 6 6 Electrons In Its Outermost Shell.
Oxygen can form two single bonds because it has six valent electrons on its outer shell. Web carbon will form four covalent bonds, nitrogen will form three covalent bonds, oxygen will form two covalent bonds, and hydrogen will form one covalent. Those are the outermost electrons,. Web a covalent bond is formed by two shared electrons.
Atoms Of Different Elements Will Form Either One,.
The number of covalent bonds that an atom can form is dictated by how many valence electrons it has. Web in order to form a covalent bond, each element has to share one unpaired electron. Web carbon will form four covalent bonds, nitrogen will form three covalent bonds, oxygen will form two covalent bonds, and hydrogen will form one covalent. Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds.
Web Oxygen And Other Atoms In Group 6A (16) Obtain An Octet By Forming Two Covalent Bonds.
To fill its valence shell, oxygen needs two additional electrons, and hydrogen needs one. For example, the hydrogen molecule, h 2, contains a covalent bond. It is easier for an oxygen atom to accept or share two. Web therefore, oxygen can form 2 single covalent bonds.
This Is False That Oxygen Atom Can Form Four Covalent Bonds With Up To Four Other Atoms.
Web oxygen is a group via element. Don’t get confused as in this question single oxygen atom (o) is asked not for dioxygen o2 o 2. Web test 1 2 3 4 5 covalent bonds forming a covalent bond a covalent bond is formed when two atoms share a pair of electrons. All the elements try to attain the stable octet configuration either by losing, gaining or sharing the valence.