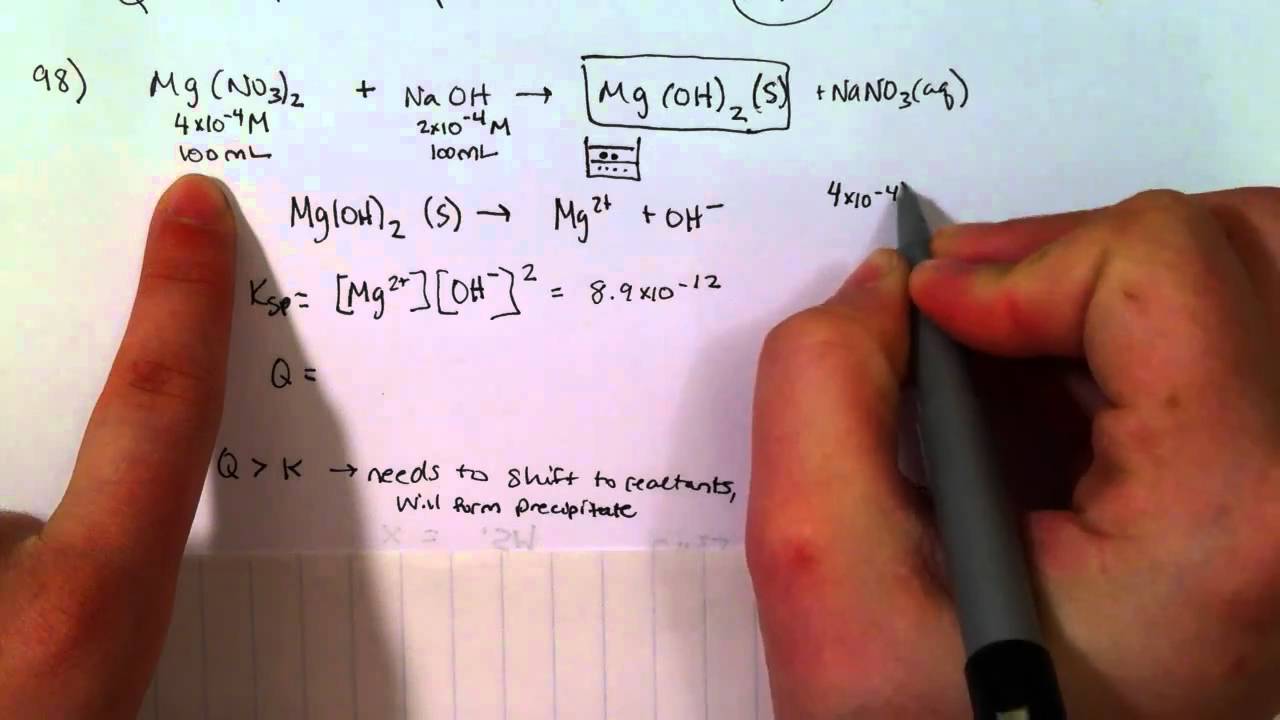
How To Determine If A Precipitate Will Form
How To Determine If A Precipitate Will Form - Thermodynamic considerations a necessary condition to the formation of precipitation is that the air becomes saturated. And this reaction produces 18.7 g precipitate. In this scenario, an equilibrium will be established to form a precipitate with. Note that precipitation may not happen immediately if q is equal to or greater than ksp. Of 6.37 for the first deprotonation of carbonic acid ( ), what is the ratio of. Calculate the initial concentrations the. This was a simple calculation mistake.the problem setup is still correct. Web goes over an example problem about predicting if a precipitate will form or not. Web 7.3k views 2 years ago solutions. If the product of the concentrations of ions after mixing is greater than the ksp value, then a.
If a precipitate is formed when a chemical reacts with lead, for. Web if the rules state that an ion is soluble, then it remains in its aqueous ion form. Note that precipitation may not happen immediately if q is equal to or greater than ksp. Web when two reactants in solution react and one or more of the products is insoluble or forms a precipitate, the reaction is called a precipitation reaction. If the product of the concentrations of ions after mixing is greater than the ksp value, then a. Thermodynamic considerations a necessary condition to the formation of precipitation is that the air becomes saturated. A solution could be supersaturated for. In this scenario, an equilibrium will be established to form a precipitate with. If an ion is insoluble based on the solubility rules, then it forms a solid with an ion from. Calculate the initial concentrations the.
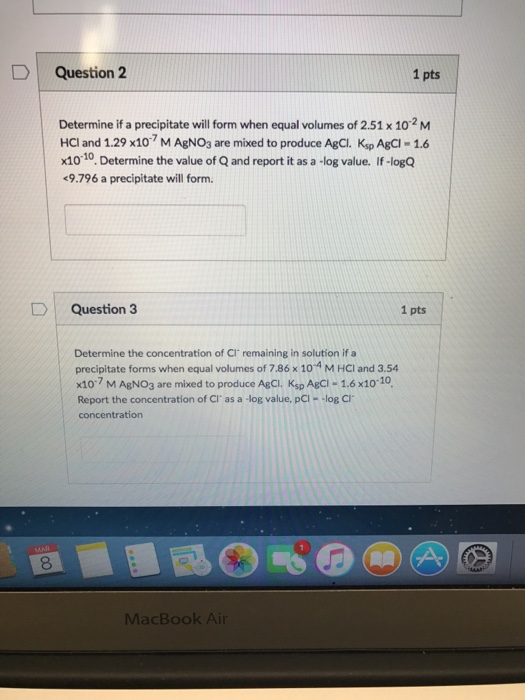
Of 6.37 for the first deprotonation of carbonic acid ( ), what is the ratio of. Web precipitation reactions are useful in determining whether a certain element is present in a solution. Note that precipitation may not happen immediately if q is equal to or greater than ksp. Calculate the initial concentrations the. If q is less than kₛₚ,. Web up to 6% cash back how many grams of precipitate form? Web how to calculate the ionic product and use it to predict whether a precipitate will form in solution. Thermodynamic considerations a necessary condition to the formation of precipitation is that the air becomes saturated. Web 7.3k views 2 years ago solutions. In this scenario, an equilibrium will be established to form a precipitate with.
Q Will a Precipitate Form (Z.15.97) YouTube
Web how to calculate the ionic product and use it to predict whether a precipitate will form in solution. Web we can use the reaction quotient to predict whether a precipitate will form when two solutions containing dissolved ionic compounds are mixed. Calculate the initial concentrations the. Determining if a precipitate will form in a solution | chemistry with cat.
12 use Ksp to find if a precipitate will form YouTube
Web 7.3k views 2 years ago solutions. Thermodynamic considerations a necessary condition to the formation of precipitation is that the air becomes saturated. A solution could be supersaturated for. If an ion is insoluble based on the solubility rules, then it forms a solid with an ion from. The molarity of cacl2 should be.00100 m instead of 0.00050 m.
How to Determine if Precipitate will Form or Not Examples, Practice
Web up to 6% cash back how many grams of precipitate form? Note that precipitation may not happen immediately if q is equal to or greater than ksp. Web to determine whether a precipitate will form, we must calculate the ionic concentrations at the moment of mixing. Calculate the initial concentrations the. Of 6.37 for the first deprotonation of carbonic.
How To Determine Solubility sharedoc
Ammonium (nh 4+) compounds are soluble. Calculate the initial concentrations the. Web if q > ksp, a precipitate will form. Of 6.37 for the first deprotonation of carbonic acid ( ), what is the ratio of. Thermodynamic considerations a necessary condition to the formation of precipitation is that the air becomes saturated.
How to determine whether a precipitate will form or not using Ksp and Q
Calculate the initial concentrations the. The molarity of cacl2 should be.00100 m instead of 0.00050 m. And this reaction produces 18.7 g precipitate. Web goes over an example problem about predicting if a precipitate will form or not. Determining if a precipitate will form in a solution | chemistry with cat a precipitate can form 2 ways:
Precipitation Reaction a reaction that results in the formation of an
If q is less than kₛₚ,. Web to determine whether a precipitate will form, we must calculate the ionic concentrations at the moment of mixing. If the product of the concentrations of ions after mixing is greater than the ksp value, then a. Web if the rules state that an ion is soluble, then it remains in its aqueous ion.
SOLVEDCalculate whether a precipitate will form
Thermodynamic considerations a necessary condition to the formation of precipitation is that the air becomes saturated. Web precipitation reactions are useful in determining whether a certain element is present in a solution. Web when two reactants in solution react and one or more of the products is insoluble or forms a precipitate, the reaction is called a precipitation reaction. In.
Determining Amount of Precipitate YouTube
Determining if a precipitate will form in a solution | chemistry with cat a precipitate can form 2 ways: If the product of the concentrations of ions after mixing is greater than the ksp value, then a. Note that precipitation may not happen immediately if q is equal to or greater than ksp. The molarity of cacl2 should be.00100 m.
Solved Determine If A Precipitate Will Form When Equal Vo...
Thermodynamic considerations a necessary condition to the formation of precipitation is that the air becomes saturated. Web precipitation reactions are useful in determining whether a certain element is present in a solution. Web to determine whether a precipitate will form, we must calculate the ionic concentrations at the moment of mixing. A solution could be supersaturated for. This was a.
Complete the table below by deciding whet... Physical Chemistry
If the product of the concentrations of ions after mixing is greater than the ksp value, then a. The molarity of cacl2 should be.00100 m instead of 0.00050 m. Web up to 6% cash back how many grams of precipitate form? And this reaction produces 18.7 g precipitate. Thermodynamic considerations a necessary condition to the formation of precipitation is that.
Web 7.3K Views 2 Years Ago Solutions.
Web when two reactants in solution react and one or more of the products is insoluble or forms a precipitate, the reaction is called a precipitation reaction. Web to determine whether a precipitate will form, we must calculate the ionic concentrations at the moment of mixing. Ammonium (nh 4+) compounds are soluble. If a precipitate is formed when a chemical reacts with lead, for.
A Solution Could Be Supersaturated For.
Web if the rules state that an ion is soluble, then it remains in its aqueous ion form. Calculate the initial concentrations the. Web up to 6% cash back how many grams of precipitate form? If an ion is insoluble based on the solubility rules, then it forms a solid with an ion from.
If The Product Of The Concentrations Of Ions After Mixing Is Greater Than The Ksp Value, Then A.
Web how to calculate the ionic product and use it to predict whether a precipitate will form in solution. Of 6.37 for the first deprotonation of carbonic acid ( ), what is the ratio of. And this reaction produces 18.7 g precipitate. Thermodynamic considerations a necessary condition to the formation of precipitation is that the air becomes saturated.
This Was A Simple Calculation Mistake.the Problem Setup Is Still Correct.
Web precipitation reactions are useful in determining whether a certain element is present in a solution. Web if q > ksp, a precipitate will form. Web goes over an example problem about predicting if a precipitate will form or not. In this scenario, an equilibrium will be established to form a precipitate with.