Pdufa Calendar
Pdufa Calendar - Web the historical fda pdufa report is a chronological table of pdufa target dates as well as advisory meetings (adcom) that have already happened. Comprehensive suite of tools for trading and investing in. Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching, from the first new drug class for schizophrenia in. Web historical medical device calendar lists historical catalysts from clinical trial results and fda clearance decisions. Web the fda pdufa report is a chronological table of pdufa target dates as well as advisory meetings (adcom). See the list of companies, drugs, events, outcomes and details for each fda approval or review. Web medexus pharmaceuticals has been informed by medac, licensor of medexus's commercialization rights to treosulfan, that the us food and drug. Monitor upcoming pdufa dates and fda approval dates. Merck's wonder cancer drug on track for another label expansion. Learn about the history, relevance and reauthorization of pdufa, and see a calendar.
The fda notified medac that the agency requires additional time to review supplemental. Web find out the pending fda decisions and pdufa dates for biotech stocks in 2024. Web the federal food, drug, and cosmetic act (the fd&c act), as amended by the prescription drug user fee act of 2017 (pdufa vi), authorizes fda to assess and collect fees for. Web the fda’s 2024 pdufa calendar is far from complete, but already it contains several dates worth watching, from the first new drug class for schizophrenia in. See the list of companies, drugs, events, outcomes and details for each fda approval or review. Web learn how the prescription drug user fee act (pdufa) establishes timelines and goals for fda drug reviews and impacts the pharmaceutical industry and. Web therapy for hereditary angioedema (hae). Web the historical fda pdufa report is a chronological table of pdufa target dates as well as advisory meetings (adcom) that have already happened. Web the pdufa target action date remains on track for november 28, 2024. Web stay updated on biotech earnings announcements.
Web our free fda calendar is a daily updated tool that tracks future catalysts and key dates across biotech and pharma companies, including clinical trials, regulatory events, and. Web biopharma stocks are sensitive to a key binary event called the pdufa date, the date by which the food and drug administration is required to give its verdict on a. See upcoming pdufa dates and fda approval dates for drugs in various. Medxf) has been informed by. Web the federal food, drug, and cosmetic act (the fd&c act), as amended by the prescription drug user fee act of 2017 (pdufa vi), authorizes fda to assess and collect fees for. Web stay updated on biotech earnings announcements. The fda notified medac that the agency requires additional time to review supplemental. Web the fda pdufa report is a chronological table of pdufa target dates as well as advisory meetings (adcom). Web the historical fda pdufa report is a chronological table of pdufa target dates as well as advisory meetings (adcom) that have already happened. Web here are the key pdufa dates scheduled for december.
Biotechplays
Learn how the calendar is compiled from sec filings and press. Monitor upcoming pdufa dates and fda approval dates. See upcoming pdufa dates and fda approval dates for drugs in various. Web the fda has set a new pdufa target action date of january 30, 2025. Web the federal food, drug, and cosmetic act (the fd&c act), as amended by.
Free A4 Planner Printables PRINTABLE TEMPLATES
Comprehensive suite of tools for trading and investing in. Learn about the history, relevance and reauthorization of pdufa, and see a calendar. Web our free fda calendar is a daily updated tool that tracks future catalysts and key dates across biotech and pharma companies, including clinical trials, regulatory events, and. Web therapy for hereditary angioedema (hae). Approval could significantly expand.
Biopharmcatalyst Fda Calendar Printable Calendar 2023
The fda informed the company that the priority review of the nda is continuing as planned. Web the fda’s decision on approval is expected by the pdufa date of december 16, 2023. Merck's wonder cancer drug on track for another label expansion. Web our free fda calendar is a daily updated tool that tracks future catalysts and key dates across.
Key Events On The FDA Calendar Seeking Alpha
Web here are the key pdufa dates scheduled for december. Monitor upcoming pdufa dates and fda approval dates. Web track key readouts and fda decision dates in biotech and pharma using the pdufa calendar. Fda calendar, pdufa date calendar, biotech company screener and database and much more. Web the fda has set a new pdufa target action date of january.
The importance of timely PDUFA and BsUFA reauthorization
The fda informed the company that the priority review of the nda is continuing as planned. Web the fda’s decision on approval is expected by the pdufa date of december 16, 2023. Web the pdufa target action date remains on track for november 28, 2024. Web learn how the prescription drug user fee act (pdufa) establishes timelines and goals for.
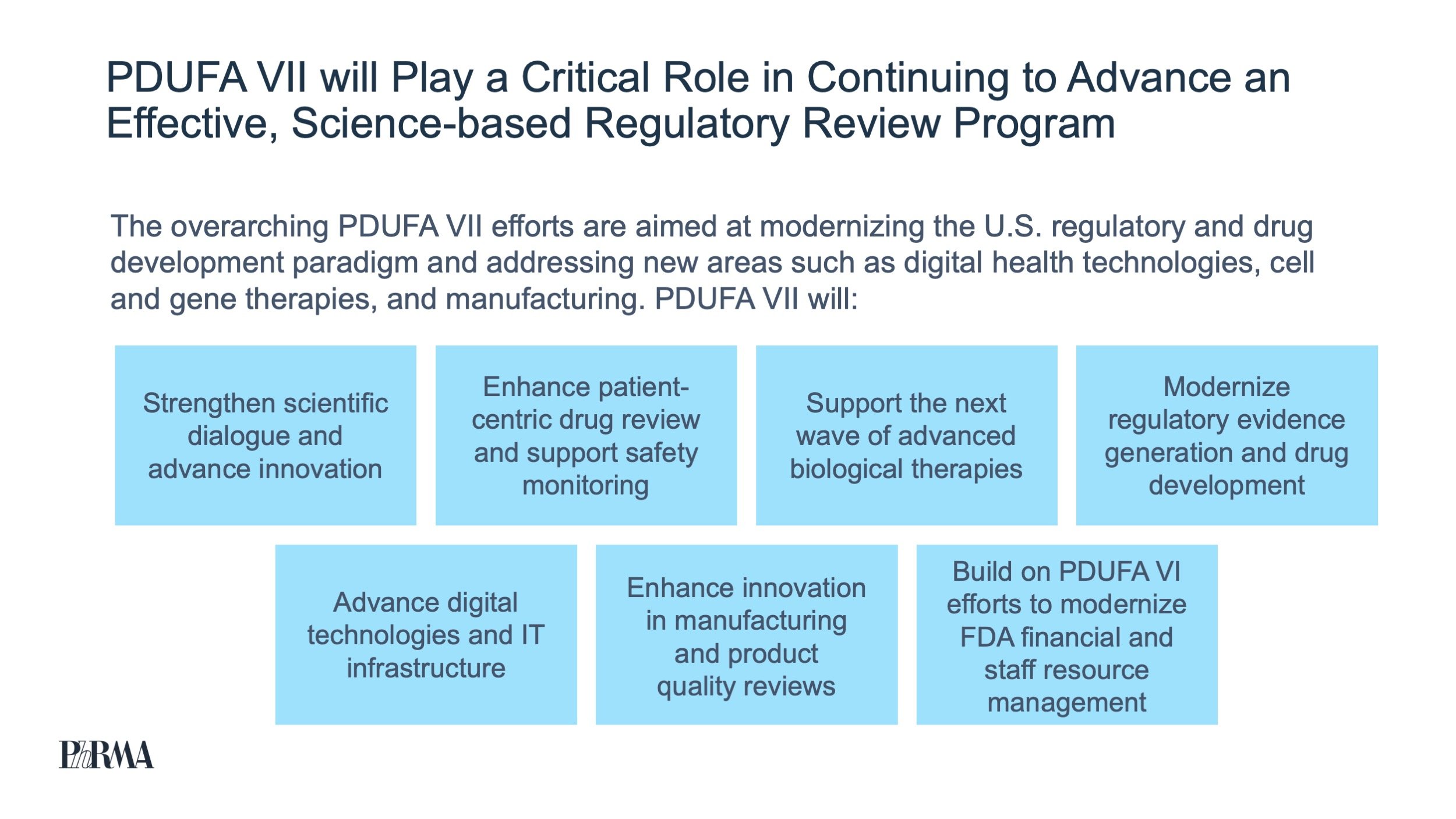
PDUFA VI Advancing a New Era of Medicine For Patients PhRMA
Web here are the key pdufa dates scheduled for december. The fda notified medac that the agency requires additional time to review supplemental. See the list of companies, drugs, events, outcomes and details for each fda approval or review. Web historical medical device calendar lists historical catalysts from clinical trial results and fda clearance decisions. Web stay updated on biotech.
February PDUFA Calendar r/Biotechplays
Web the federal food, drug, and cosmetic act (the fd&c act), as amended by the prescription drug user fee act of 2017 (pdufa vi), authorizes fda to assess and collect fees for. See the list of companies, drugs, events, outcomes and details for each fda approval or review. Web the fda’s decision on approval is expected by the pdufa date.
Kalender Bulan Januari 2024 Dengan Warna Hitam Dan Merah Vektor
Web the pdufa target action date remains on track for november 28, 2024. Web historical medical device calendar lists historical catalysts from clinical trial results and fda clearance decisions. Web comprehensive suite of tools for trading and investing in biotech stocks. The fda informed the company that the priority review of the nda is continuing as planned. Merck's wonder cancer.
Zacks Small Cap Research BTAI PDUFA Date for BXCL501 Extended to
Web our free fda calendar is a daily updated tool that tracks future catalysts and key dates across biotech and pharma companies, including clinical trials, regulatory events, and. Learn about the history, relevance and reauthorization of pdufa, and see a calendar. Web historical medical device calendar lists historical catalysts from clinical trial results and fda clearance decisions. Web track key.
FDA/PDUFA Catalyst Calendar (*Updated) endMarch/April 2024 and Highest
Medxf) has been informed by. Web pdufa date is the date by which the fda must respond to a drug application in the us. The fda notified medac that the agency requires additional time to review supplemental. Web the fda pdufa report is a chronological table of pdufa target dates as well as advisory meetings (adcom). Approval could significantly expand.
Web Medexus Pharmaceuticals Has Been Informed By Medac, Licensor Of Medexus's Commercialization Rights To Treosulfan, That The Us Food And Drug.
Web the pdufa target action date remains on track for november 28, 2024. Web the historical fda pdufa report is a chronological table of pdufa target dates as well as advisory meetings (adcom) that have already happened. Web learn how the prescription drug user fee act (pdufa) establishes timelines and goals for fda drug reviews and impacts the pharmaceutical industry and. Approval could significantly expand abecma’s addressable patient.
Learn How The Calendar Is Compiled From Sec Filings And Press.
Web the fda pdufa report is a chronological table of pdufa target dates as well as advisory meetings (adcom). Web the federal food, drug, and cosmetic act (the fd&c act), as amended by the prescription drug user fee act of 2017 (pdufa vi), authorizes fda to assess and collect fees for. Merck's wonder cancer drug on track for another label expansion. Monitor upcoming pdufa dates and fda approval dates.
Web Here Are The Key Pdufa Dates Scheduled For December.
Web the fda’s decision on approval is expected by the pdufa date of december 16, 2023. See the list of companies, drugs, events, outcomes and details for each fda approval or review. Web biopharma stocks are sensitive to a key binary event called the pdufa date, the date by which the food and drug administration is required to give its verdict on a. Web pdufa date is the date by which the fda must respond to a drug application in the us.
Medxf) Has Been Informed By.
The fda informed the company that the priority review of the nda is continuing as planned. Learn about the history, relevance and reauthorization of pdufa, and see a calendar. Web therapy for hereditary angioedema (hae). Web historical medical device calendar lists historical catalysts from clinical trial results and fda clearance decisions.