Printable Polyatomic Ions List
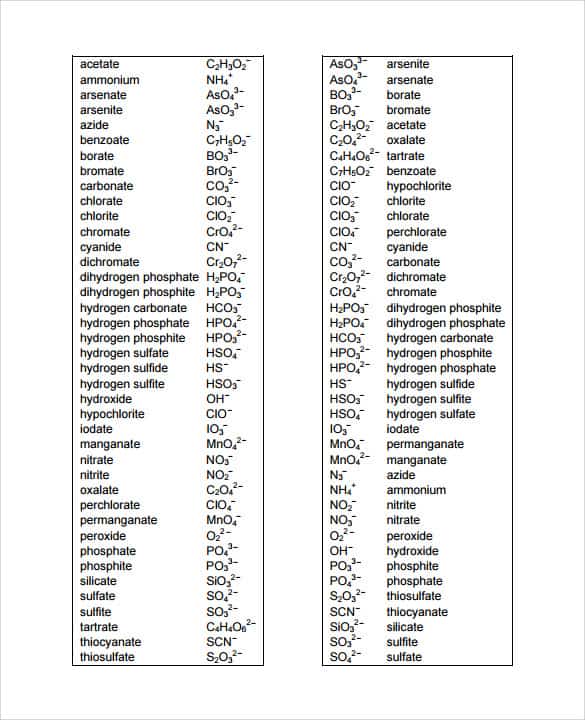
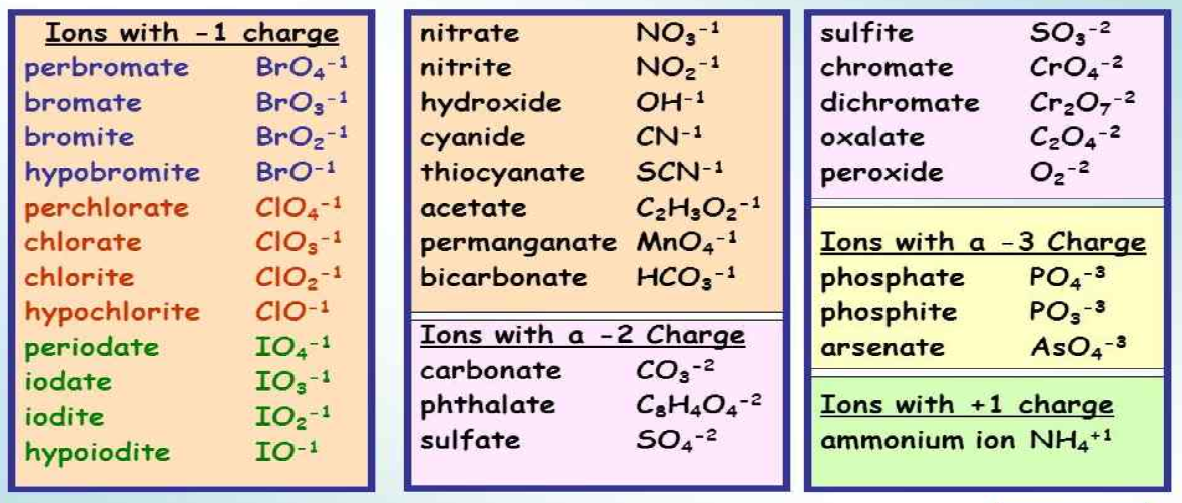
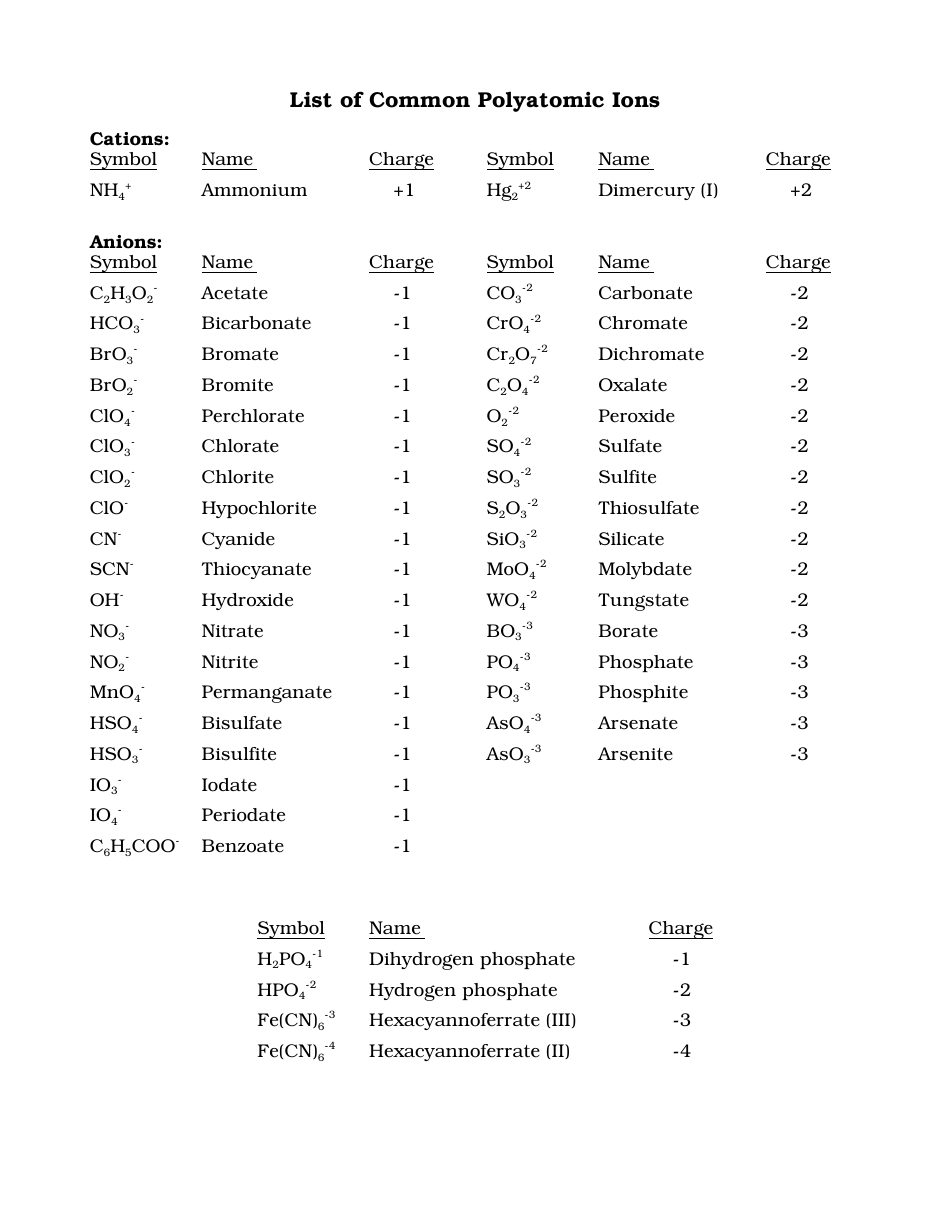
Printable Polyatomic Ions List - Web some polyatomic ions can be changed by change number of oxygens. Here's a guide to some of the most common examples! For example, \(\ce{no3^{−}}\) is the nitrate ion; Memorize all names and formulas on back. Web name and formulas of common polyatomic ions no3 − nitrate ion c 2h3o2 − acetate ion no2 − nitrite ion cn− cyanide ion nh4 + ammonium ion c 2o4 2− oxalate ion so4 2− sulfate ion clo 4 − perchlorate ion so3 2− sulfite ion clo 3 − chlorate ion hso4 − hydrogen sulfate ion clo 2 − chlorite ion hso3 − hydrogen sulfite ion clo−. Figure \(\pageindex{2}\) lists the most common polyatomic ions. There are 4 exercises to practice, plus complete instructions, in the 5 page packet. Web use this naming polyatomic ions list and worksheet (answers provided) to quickly learn important chemical names and formulas. Each entry contains the ion's name, molecular formula and chemical structure. Web this document lists and describes many common polyatomic ions, including their chemical formulas and charge.
Web this is a list of some of the most common polyatomic ions. Web the common polyatomic ions chart provides a list of common ions with their respective formulas. It is used to help identify and write the formulas for these ions in chemical compounds. It also provides some rules for naming polyatomic ions based on adding or removing oxygen and hydrogen atoms. C 4h 4o 6 2⎯ tartrate : Each entry contains the ion's name, molecular formula and chemical structure. Web some polyatomic ions can be changed by change number of oxygens. Here's a guide to some of the most common examples! Clo 3 ⎯ hypochlorite : Here's a guide to some of the most common examples!
For example, \(\ce{no3^{−}}\) is the nitrate ion; Ammonium acetate carbonate chlorate chlorite chromate cyanide. Co 3 2⎯ carbonate : There are 4 exercises to practice, plus complete instructions, in the 5 page packet. Web table \(\pageindex{2}\) lists the ion names and ion formulas of the most common polyatomic ions. Memorize all of the polyatomic ions: Here's a guide to some of the most common examples! It provides a list of these ions, along with their chemical formulas and charges. Web this document lists and describes many common polyatomic ions, including their chemical formulas and charge. Here's a guide to some of the most common examples!
Polyatomic ions Pearltrees
C 2h 3o 2⎯ acetate : Cro 4 2⎯ chromate : C 4h 4o 6 2⎯ tartrate : C 7h 5o 2⎯ benzoate : Clo 3 ⎯ hypochlorite :
5+ Free Polyatomic Ion Charts Word Excel Fomats
Memorize all of the binary compounds: The common polyatomic ions chart or tables is used for reference and identification of common polyatomic ions. While there are many such ions in the world, you are responsible for knowing the ions listed in the following tables. Aso 3 3 ⎯ arsenite. Here's a guide to some of the most common examples!
polyatomic ions Chemistry education, High school science, Secondary
It is used to help identify and write the formulas for these ions in chemical compounds. For example, \(\ce{no3^{−}}\) is the nitrate ion; C 2h 3o 2⎯ acetate : There are 4 exercises to practice, plus complete instructions, in the 5 page packet. Memorize all of the binary compounds:
Common Polyatomic Ions List Download Printable PDF Templateroller
Web these polyatomic ions are extremely common in chemistry and thus it is important to be able to both recognize and name them. Figure \(\pageindex{2}\) lists the most common polyatomic ions. C 4h 4o 6 2⎯ tartrate : A polyatomic ion is a charged species consisting of two or more atoms covalently bonded together. It provides a list of these.
Polyatomic Ions List and Worksheet Easy Hard Science
Web this is a list of some of the most common polyatomic ions. Here's a guide to some of the most common examples! Cr 2o 7 2⎯ dichromate : Memorize all of the polyatomic ions: Names, formulae & charges a polyatomic ion is a charged species consisting of two or more atoms covalently bonded together.
Polyatomic Ion Charts Find Word Templates
It also provides some rules for naming polyatomic ions based on adding or removing oxygen and hydrogen atoms. C 4h 4o 6 2⎯ tartrate : Figure \(\pageindex{2}\) lists the most common polyatomic ions. Web this is a list of some of the most common polyatomic ions. Web use this naming polyatomic ions list and worksheet (answers provided) to quickly learn.
Polyatomic Ions List Free Download
Memorize all names and formulas on back. Figure \(\pageindex{2}\) lists the most common polyatomic ions. C 4h 4o 6 2⎯ tartrate : Web this is a list of some of the most common polyatomic ions. Each entry contains the ion's name, molecular formula and chemical structure.
Polyatomic Ions Naming and Formulas Study Guide Inspirit Learning Inc
Memorize all of the polyatomic ions: It also provides some rules for naming polyatomic ions based on adding or removing oxygen and hydrogen atoms. Bo 3 3⎯ borate : C 2o 4 2⎯ oxalate : While there are many such ions in the world, you are responsible for knowing the ions listed in the following tables.
Common Polyatomic Ions Chart Cations, Anions Download Printable PDF
Web table \(\pageindex{1}\) lists the ion names and ion formulas of the most common polyatomic ions. A polyatomic ion is a charged species consisting of two or more atoms covalently bonded together. C 4h 4o 6 2⎯ tartrate : It has one nitrogen atom and three oxygen atoms and an overall 1− charge. Memorize all names and formulas on back.
Standard Polyatomic Ions Chart Free Download
It has one nitrogen atom and three oxygen atoms and an overall 1− charge. Web this polyatomic ions list contains many common polyatomic ions grouped by charge. Memorize all of the binary compounds: Clo 3 ⎯ hypochlorite : Web this is a list of some of the most common polyatomic ions.
It Provides A List Of These Ions, Along With Their Chemical Formulas And Charges.
Co 3 2⎯ carbonate : Web these polyatomic ions are extremely common in chemistry and thus it is important to be able to both recognize and name them. A polyatomic ion is a charged species consisting of two or more atoms covalently bonded together. Web some polyatomic ions can be changed by change number of oxygens.
Figure \(\Pageindex{2}\) Lists The Most Common Polyatomic Ions.
Web table \(\pageindex{2}\) lists the ion names and ion formulas of the most common polyatomic ions. Web this is a list of some of the most common polyatomic ions. Memorize all of the binary compounds: Aso 3 3 ⎯ arsenite.
C 2H 3O 2⎯ Acetate :
Bo 3 3⎯ borate : Here's a guide to some of the most common examples! Each entry contains the ion's name, molecular formula and chemical structure. C 4h 4o 6 2⎯ tartrate :
The Common Polyatomic Ions Chart Or Tables Is Used For Reference And Identification Of Common Polyatomic Ions.
Ammonium acetate carbonate chlorate chlorite chromate cyanide. Memorize all names and formulas on back. Web name and formulas of common polyatomic ions no3 − nitrate ion c 2h3o2 − acetate ion no2 − nitrite ion cn− cyanide ion nh4 + ammonium ion c 2o4 2− oxalate ion so4 2− sulfate ion clo 4 − perchlorate ion so3 2− sulfite ion clo 3 − chlorate ion hso4 − hydrogen sulfate ion clo 2 − chlorite ion hso3 − hydrogen sulfite ion clo−. For example, \(\ce{no3^{−}}\) is the nitrate ion;