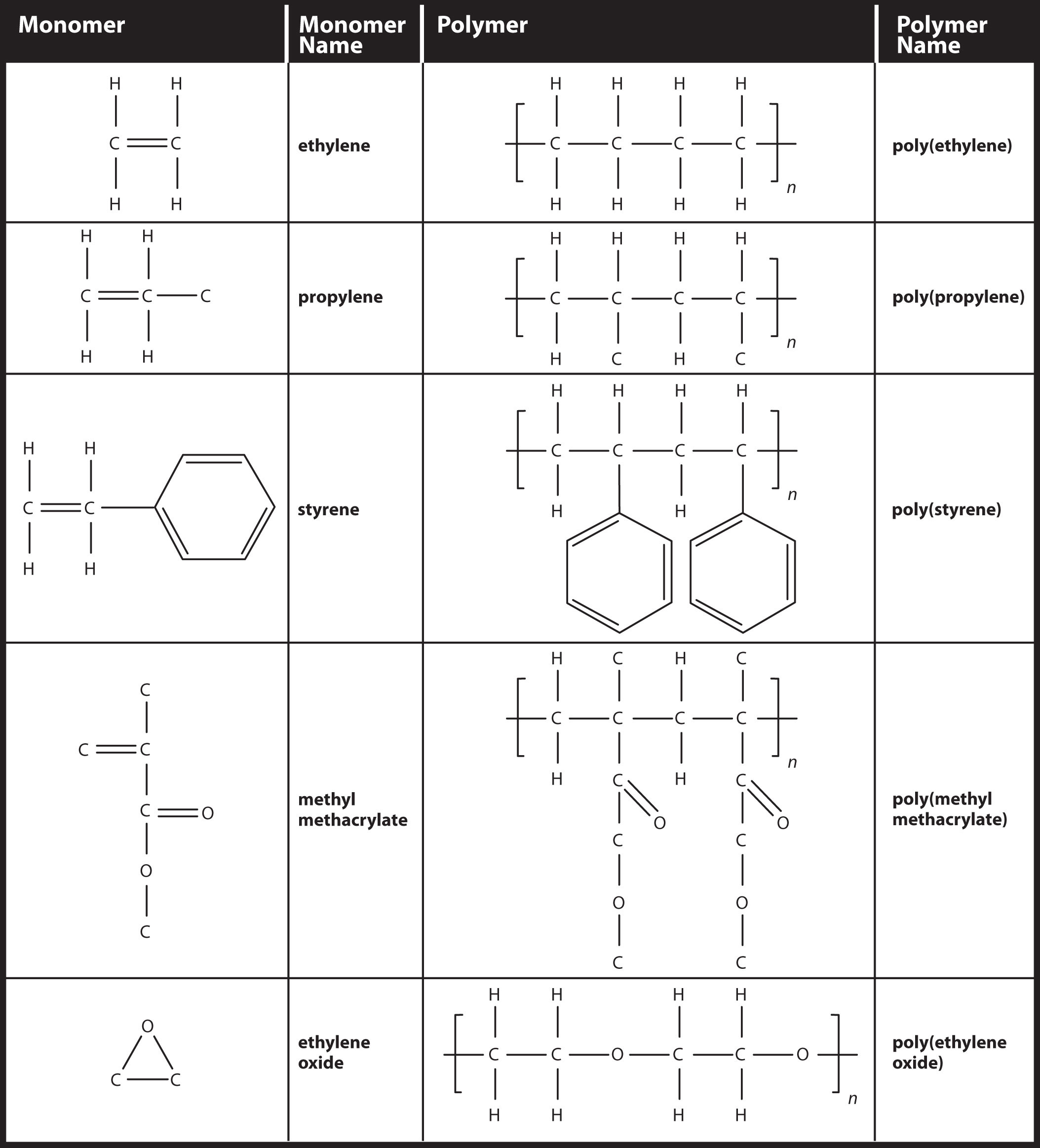
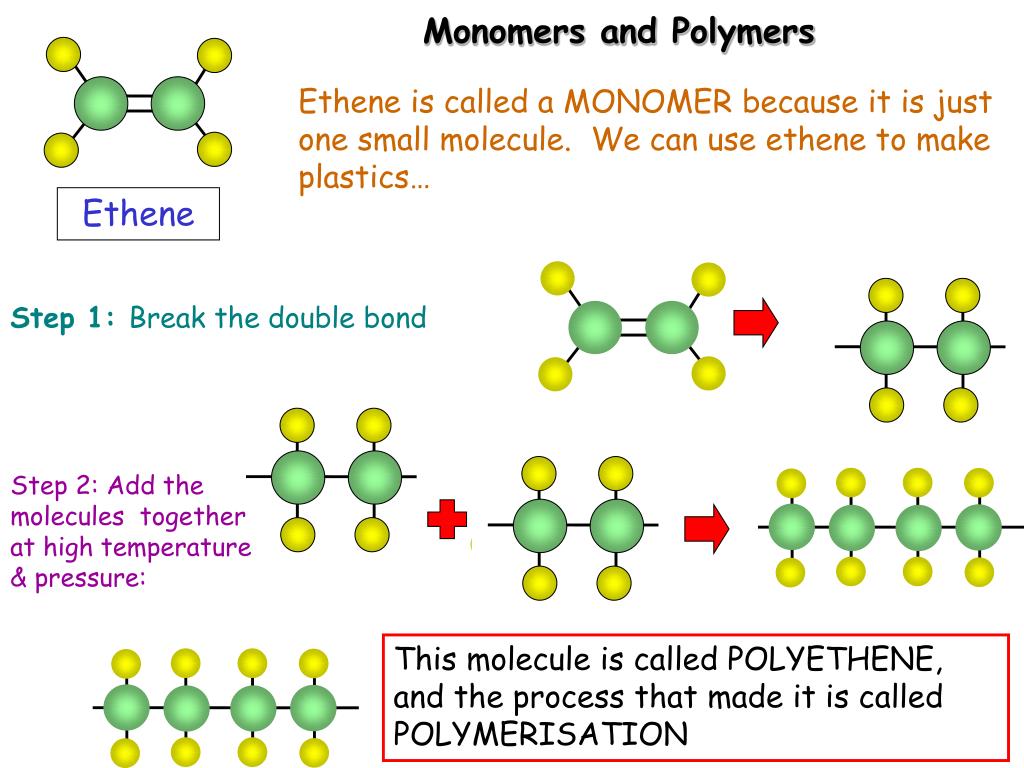
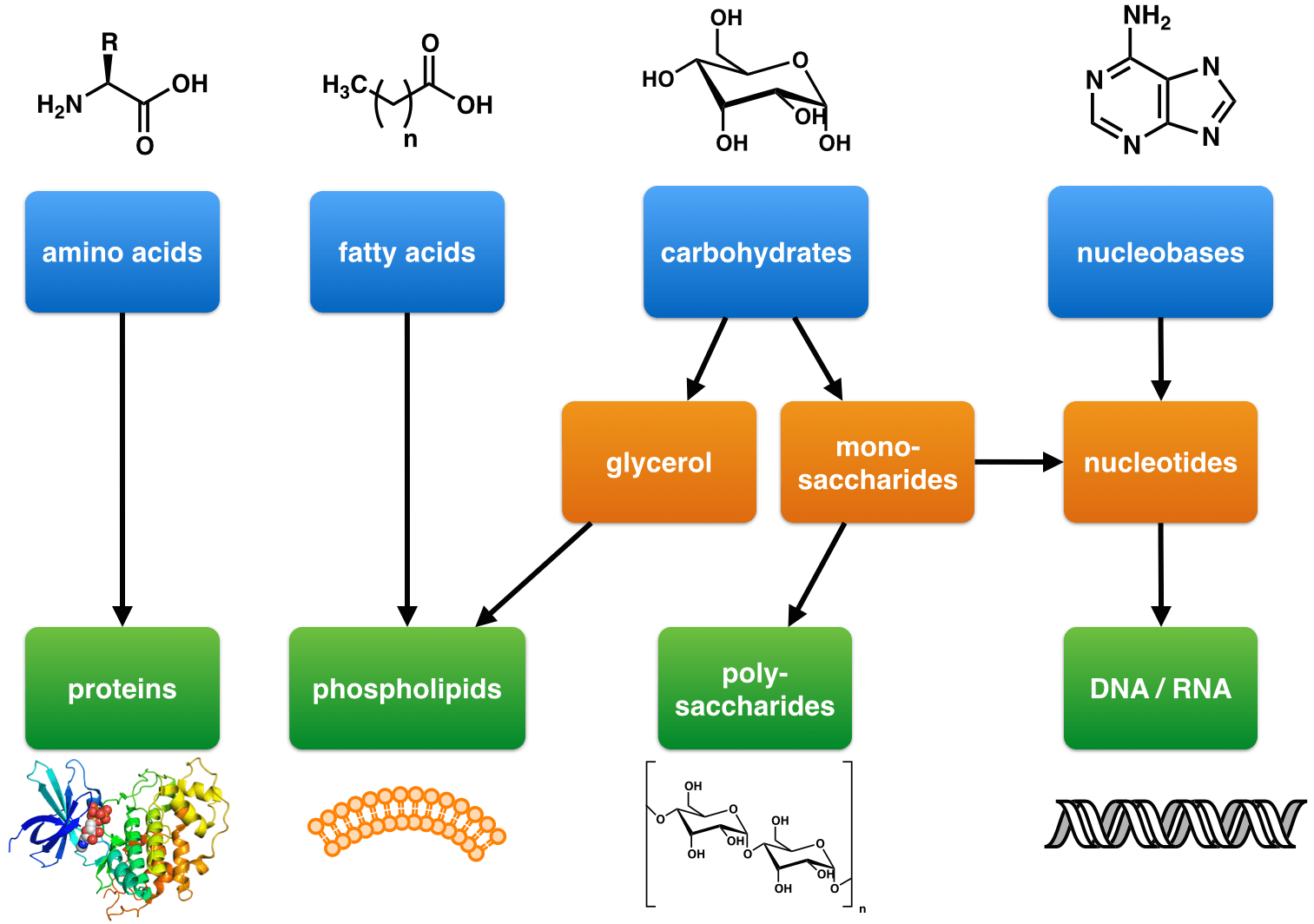
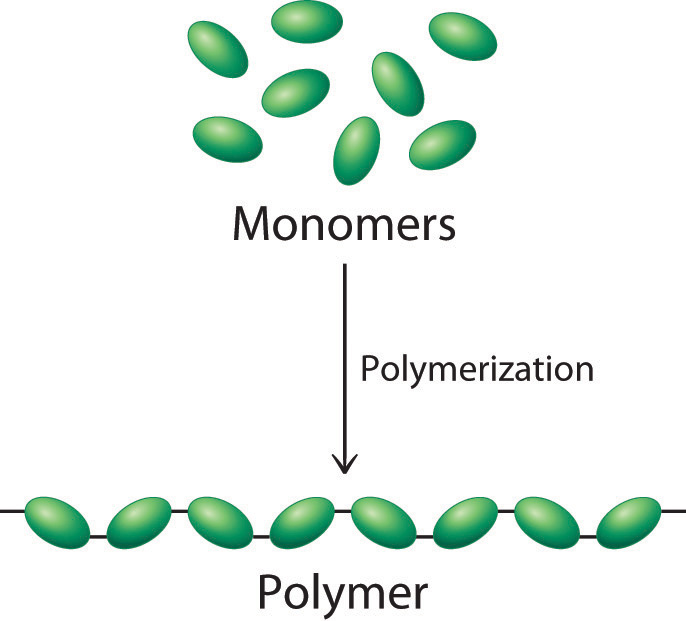
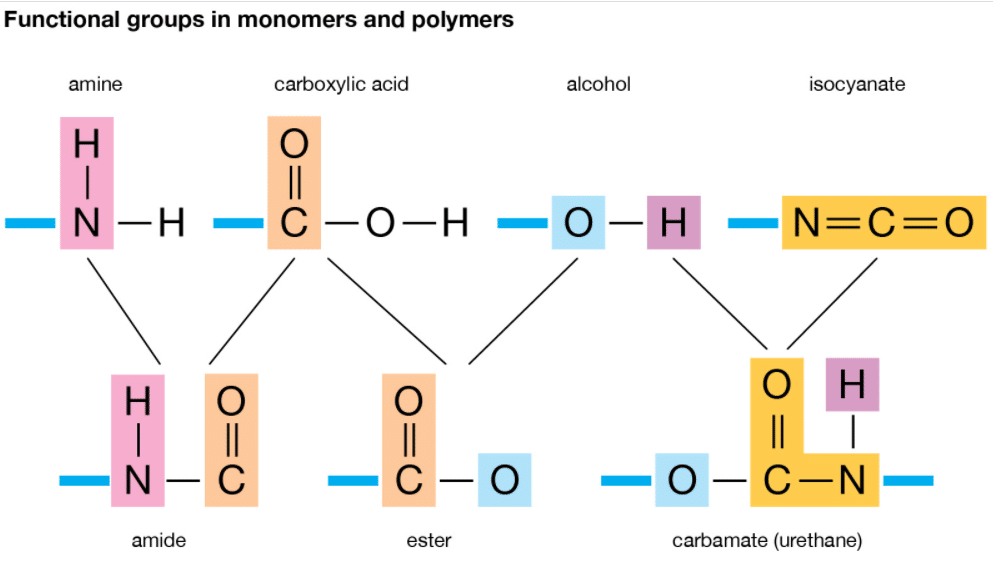
What Type Of Reaction Links Monomers Together To Form Polymers
What Type Of Reaction Links Monomers Together To Form Polymers - Web link monomers to form a polymer. The reaction that joins two monomers to form a polymer is known as a _____ reaction. Web polymers are broken down into monomers via hydrolysis reactions, in which a bond is broken, or lysed, by addition of a water molecule. Glucose is the most common natural monomer. In this type of reaction,. The monomers combine with each other using covalent bonds to form larger molecules. Polymerization reactions link monomers to polymers. Web chemistry biological chemistry monomers and polymers term 1 / 27 what is a polymer? Glucose also provides a vital source of. Click the card to flip 👆 definition 1 / 27 large carbon based macromolecules formed from.
Web spread the love the monomers combine with each other using covalent bonds to form larger molecules known as polymers. Multiple monomers together form a molecule called a polymer. Glucose also provides a vital source of. Web monomers are smaller molecules, and when bonded together, make up polymers. Web gabriel true jun 4, 2018 polymers explanation: Web chemistry biological chemistry monomers and polymers term 1 / 27 what is a polymer? In this type of reaction,. Web link monomers to form a polymer. Web most macromolecules are made from single subunits, or building blocks, called monomers. To form a polymer ,monomers are linked together by dehydrat.
Glucose is the most common natural monomer. Whenever a glycosidic bond is formed, there is the elimination. Web most macromolecules are made from single subunits, or building blocks, called monomers. It links together to form polymers of starch, cellulose and glycogen. Multiple monomers together form a molecule called a polymer. Web when two adjacent monosaccharide units link to form disaccharides or polysaccharides, a glycosidic bond is formed. Web a glycosidic bond is produced when two neighbouring monosaccharides join together to form disaccharides or polysaccharides. In this type of reaction,. The monomers combine with each other using covalent bonds to form larger molecules. What is the name of the reaction that links monomers together to form polymers?
Polymers
Multiple monomers together form a molecule called a polymer. Web monomers are smaller molecules, and when bonded together, make up polymers. Glucose is the most common natural monomer. There are numerous types of polymerization reactions: Web most macromolecules are made from single subunits, or building blocks, called monomers.
Unit 2
Web when two adjacent monosaccharide units link to form disaccharides or polysaccharides, a glycosidic bond is formed. Web how are monomers joined together to form polymers? Web monomers are smaller molecules, and when bonded together, make up polymers. Glucose is the most common natural monomer. Web gabriel true jun 4, 2018 polymers explanation:
PPT Monomers and Polymers PowerPoint Presentation, free download ID
Glucose is the most common natural monomer. Web most macromolecules are made from single subunits, or building blocks, called monomers. To form a polymer ,monomers are linked together by dehydrat. Web a glycosidic bond is produced when two neighbouring monosaccharides join together to form disaccharides or polysaccharides. There are numerous types of polymerization reactions:
Types of Monomers Sciencing
The individual smaller molecules are. Web when monomers come together to form a polymer, they link with each other through the process known as polymerization. Web when two adjacent monosaccharide units link to form disaccharides or polysaccharides, a glycosidic bond is formed. In doing so, monomers release water molecules as byproducts. It links together to form polymers of starch, cellulose.
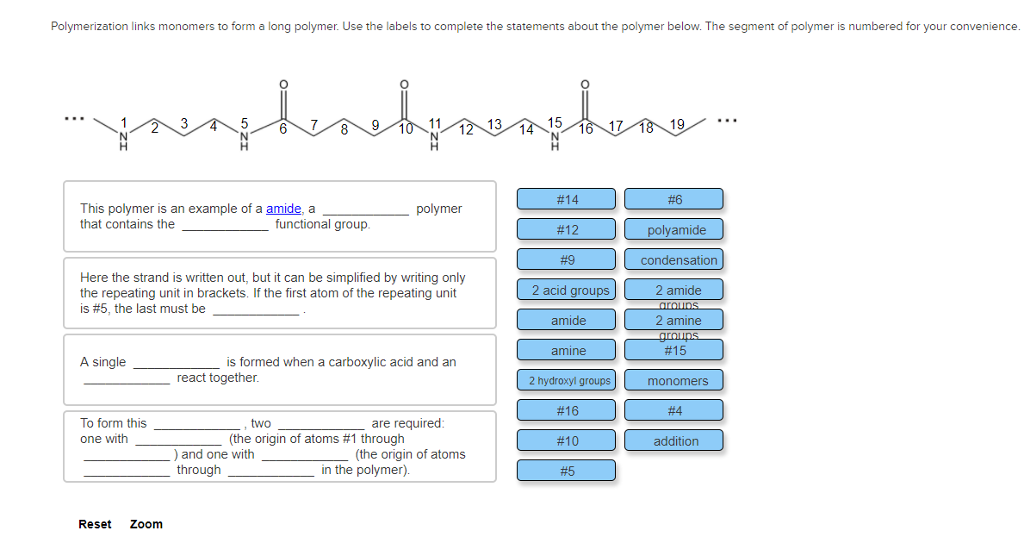
Solved Polymerization links monomers to form a long polymer.
Web how are monomers joined together to form polymers? What is the name of the reaction that links monomers together to form polymers? Web gabriel true jun 4, 2018 polymers explanation: Web monomers are smaller molecules, and when bonded together, make up polymers. There are numerous types of polymerization reactions:
How Do Macromolecules Form? — Overview & Process Expii
Web polymers are broken down into monomers via hydrolysis reactions, in which a bond is broken, or lysed, by addition of a water molecule. The molecular weight of the formed polymer is exactly the same as the sum of all monomers. The monomers combine with each other using covalent bonds to form larger molecules. It links together to form polymers.
What Is a Polymer? Live Science
To form a polymer ,monomers are linked together by dehydrat. Web chemistry biological chemistry monomers and polymers term 1 / 27 what is a polymer? In organic macromolecules (there may be other. Monomers are small molecules that can be joined to form more complex molecules called polymers in a. Web most macromolecules are made from single subunits, or building blocks,.
Types of Monomers Sciencing
Monomers are linked together by a type of reaction called a dehydration or condensation reaction. There are numerous types of polymerization reactions: In organic macromolecules (there may be other. Whenever a glycosidic bond is formed, there is the elimination. The individual smaller molecules are.
IGCSE Chemistry 2017 4.44 Know that an Addition Polymer is Formed by
Web how are monomers joined together to form polymers? Web spread the love the monomers combine with each other using covalent bonds to form larger molecules known as polymers. Web the monomers combine with each other via covalent bonds to form larger molecules known as polymers. Once linked, the process can be reversed. Glucose also provides a vital source of.
What is Polymerization? Definition, Types & Examples
In doing so, monomers release water molecules as byproducts. Web chemistry biological chemistry monomers and polymers term 1 / 27 what is a polymer? Web the monomers combine with each other via covalent bonds to form larger molecules known as polymers. In doing so, monomers release. Web when two adjacent monosaccharide units link to form disaccharides or polysaccharides, a glycosidic.
Glucose Is The Most Common Natural Monomer.
There are numerous types of polymerization reactions: Web monomers are smaller molecules, and when bonded together, make up polymers. Web spread the love the monomers combine with each other using covalent bonds to form larger molecules known as polymers. Web most macromolecules are made from single subunits, or building blocks, called monomers.
Web Link Monomers To Form A Polymer.
Multiple monomers together form a molecule called a polymer. The molecular weight of the formed polymer is exactly the same as the sum of all monomers. The monomers combine with each other using covalent bonds to form larger molecules. In this type of reaction,.
During A Hydrolysis Reaction, A.
Polymerization reactions link monomers to polymers. Glucose also provides a vital source of. Web how are monomers joined together to form polymers? Web chemistry biological chemistry monomers and polymers term 1 / 27 what is a polymer?
Click The Card To Flip 👆 Definition 1 / 27 Large Carbon Based Macromolecules Formed From.
Once linked, the process can be reversed. Web a glycosidic bond is produced when two neighbouring monosaccharides join together to form disaccharides or polysaccharides. In doing so, monomers release water molecules as byproducts. Web polymers are broken down into monomers via hydrolysis reactions, in which a bond is broken, or lysed, by addition of a water molecule.