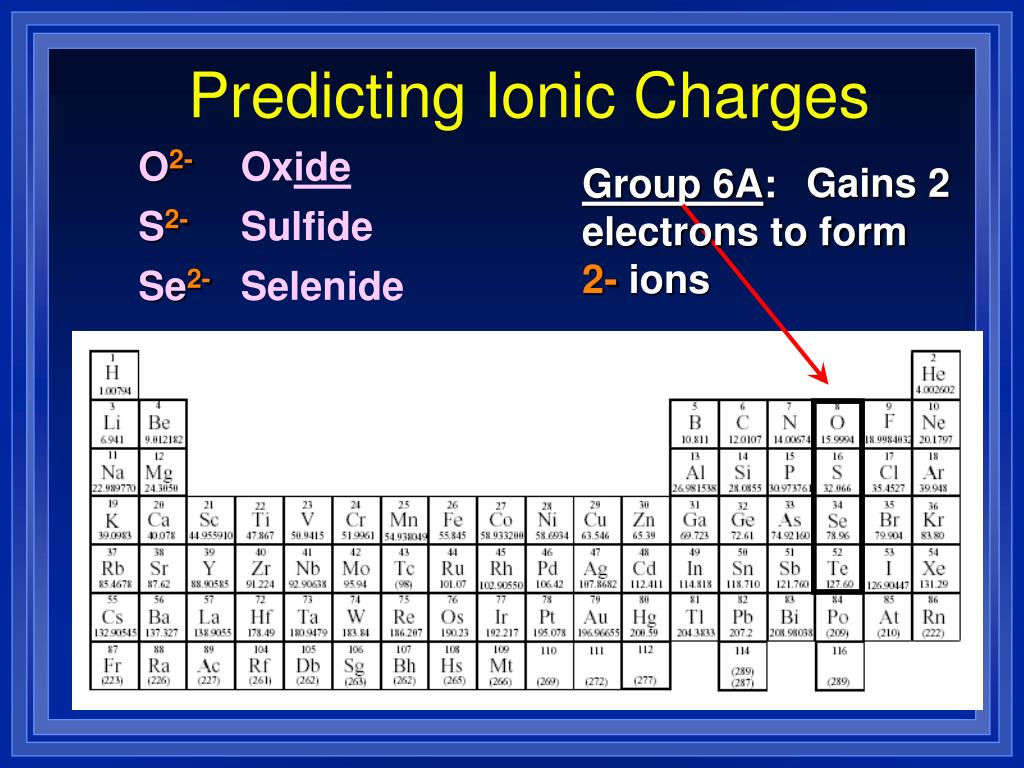
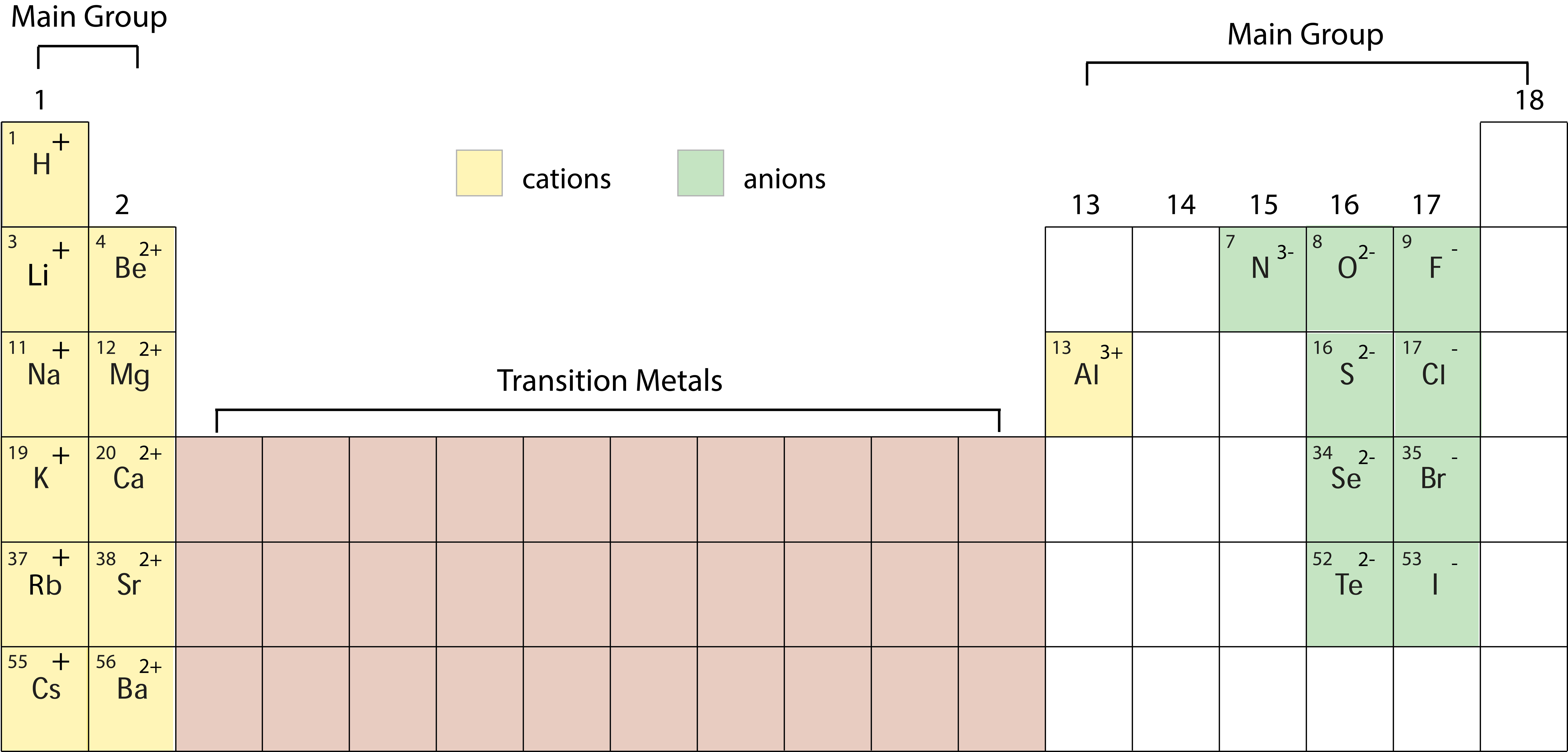
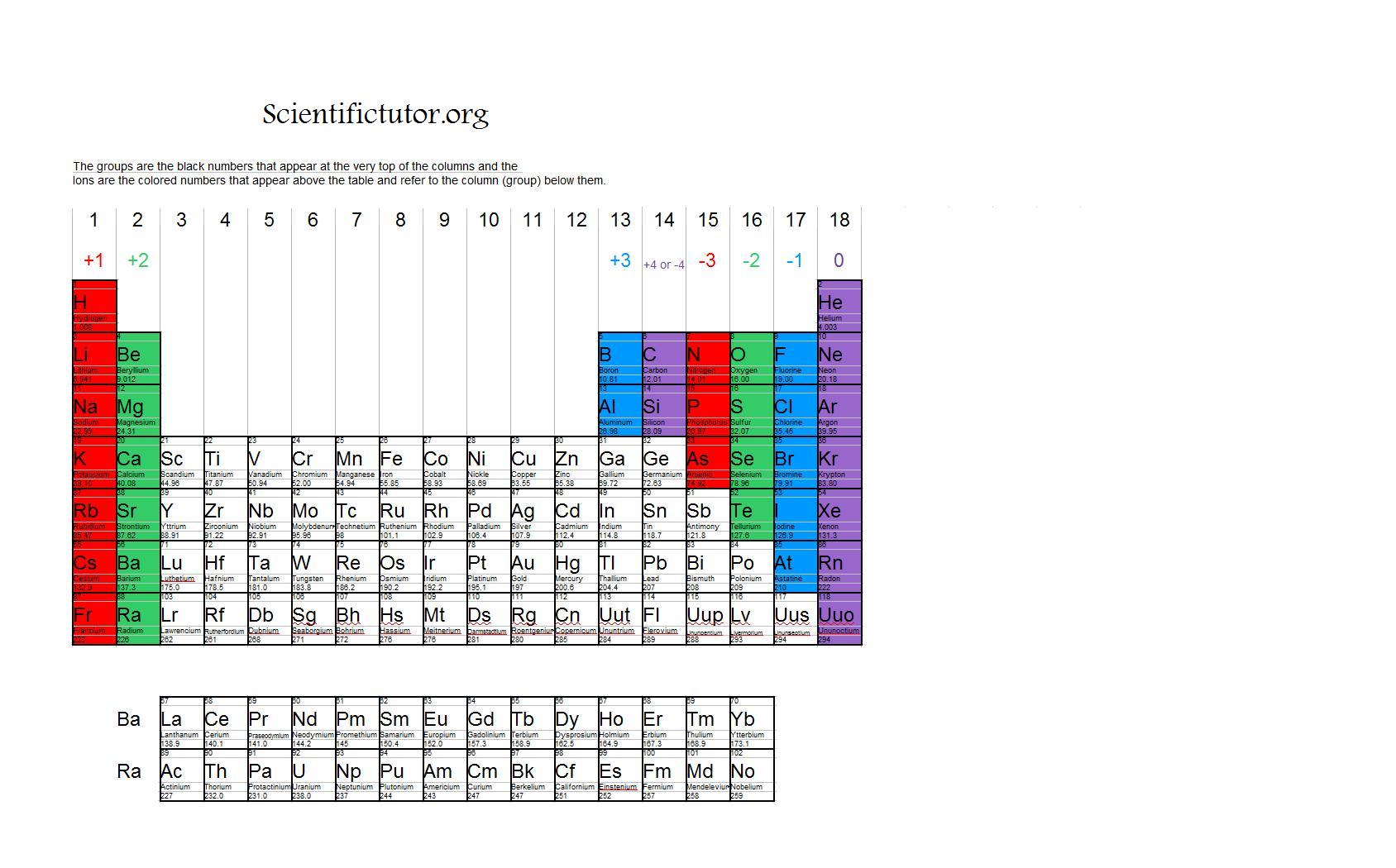
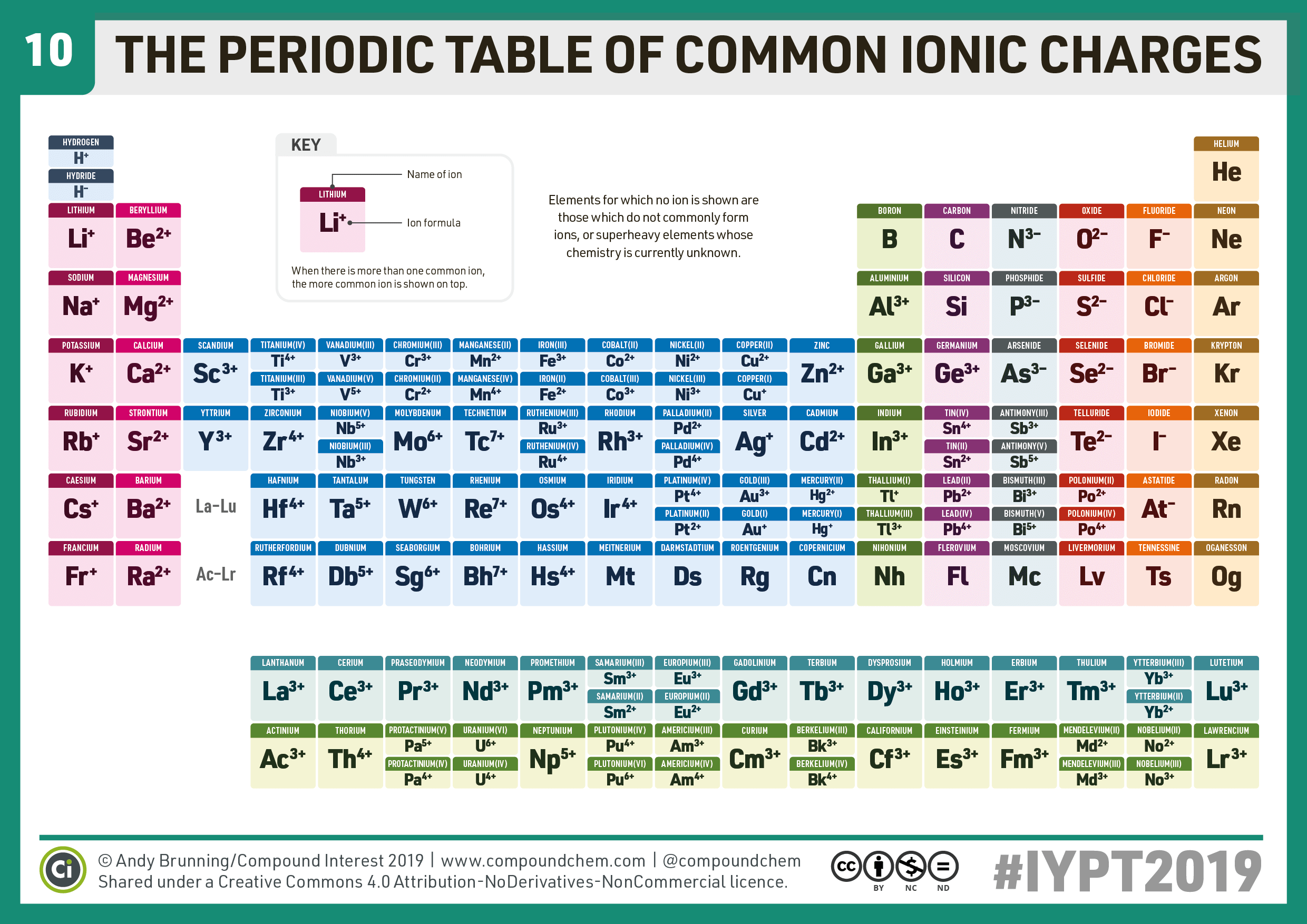
When Group 2A Elements Form Ions They
When Group 2A Elements Form Ions They - Web when group 2a elements form ions what happens? They tend to lose electrons to form positive ions. When atom lose electron positive charge is created on atom. There are two types of ions. The number of valence electrons an atom has will. Metals are electron rich substances. Web test match created by dkmoran terms in this set (47) horizontal row in the periodic table period vertical column in the periodic table group basis for mendeleev's arrangement of. It is formed when an atom loses the electrons. Some examples of group 2a elements. Using periodic properties to identify group 2a cations and group 7a anions suppose that substances \(\ce{x}\), \(\ce{y}\), and.
Anion cation 1 = anion it is. When two elements form more than one compound,. Beryllium (be), magnesium (mg), calcium (ca), strontium (sr), barium (ba), and radium (ra). The number of valence electrons an atom has will. Lose two electrons aluminum is a group 3a metal. Using periodic properties to identify group 2a cations and group 7a anions suppose that substances \(\ce{x}\), \(\ce{y}\), and. Nitride ion what is the correct name for the n³⁻ ion? It is formed when an atom loses the electrons. Some examples of group 2a elements. When atom lose electron positive charge is created on atom.
Web when group 2a elements form ions, they. Metals are electron rich substances. Some examples of group 2a elements. Using periodic properties to identify group 2a cations and group 7a anions suppose that substances \(\ce{x}\), \(\ce{y}\), and. Lose two electrons aluminum is a group 3a metal. Metals are electron rich substances. Nitride ion what is the correct name for the n³⁻ ion? The number of valence electrons an atom has will. M g → m g2+ + 2e−. They tend to lose electrons to form positive ions.
The Periodic Table and its Design Pathways to Chemistry
Nitride ion what is the correct name for the n³⁻ ion? Web in any chemical compound, the masses of elements are always in the same proportion by mass. Anion cation 1 = anion it is. When an atom loses are gain the electrons ions are formed. When naming a transition metal ion that can have more.
PPT Chapter 9 “Chemical Names and Formulas” PowerPoint Presentation
Web when group 2a elements form ions what happens? It is formed when an atom loses the electrons. When group 2a elements form ions, they lose two electrons. When two elements form more than one compound,. When an atom loses are gain the electrons ions are formed.
table of elements chart
As you know, neutral atoms become ions by losing or by gaining electrons. Some examples of group 2a elements. They tend to lose electrons to form positive ions. M g → m g2+ + 2e−. Web when group 2a elements form ions, they a.
PreChemistry
Beryllium (be), magnesium (mg), calcium (ca), strontium (sr), barium (ba), and radium (ra). When two elements form more than one compound,. Metals are electron rich substances. As you know, neutral atoms become ions by losing or by gaining electrons. They tend to lose electrons to form positive ions.
Chemistry Chp 9 Chemical Names and Formulas PowerPoint
Web group 2a (or iia) of the periodic table are the alkaline earth metals : When naming a transition metal ion that can have more. Web for elements in groups. They tend to lose electrons to form positive ions. 1, 2 and 3, the number of electrons lost is the same as the group number.
Pin on Education
X → x⁺ + e⁻. Web group 2a (or iia) of the periodic table are the alkaline earth metals : Anion cation 1 = anion it is. As you know, neutral atoms become ions by losing or by gaining electrons. 1, 2 and 3, the number of electrons lost is the same as the group number.
Chemistry Chp 9 Chemical Names and Formulas PowerPoint
Web when group 2a elements form ions, they. Nitride ion what is the correct name for the n³⁻ ion? Web when group 2a elements form ions what happens? Anion cation 1 = anion it is. Web answer they lose 2 electrons.
Chem Ions Scientific Tutor
When two elements form more than one compound,. When group 2a elements form ions, they lose two electrons. Web answer they lose 2 electrons. Nitride ion what is the correct name for the n³⁻ ion? They tend to lose electrons to form positive ions.
10 Periodic Table of Common Ions Compound Interest
Roman numeral following the name when naming a transition metal ion that can. Using periodic properties to identify group 2a cations and group 7a anions suppose that substances \(\ce{x}\), \(\ce{y}\), and. The number of valence electrons an atom has will. Web when group 2a elements form ions, they. When an atom loses are gain the electrons ions are formed.
CH103 CHAPTER 4 Ions and Ionic Compounds Chemistry
Nitride ion what is the correct name for the n³⁻ ion? When an atom loses are gain the electrons ions are formed. When group 2a elements form ions, they lose two electrons. Lose two electrons aluminum is a group 3a metal. Web test match created by dkmoran terms in this set (47) horizontal row in the periodic table period vertical.
1, 2 And 3, The Number Of Electrons Lost Is The Same As The Group Number.
Web for elements in groups. Anion cation 1 = anion it is. Some examples of group 2a elements. Web when group 2a elements form ions, they a.
Calcium Does Not Obey The Octet Rule.
Nitride ion what is the correct name for the n³⁻ ion? It does not change its number of electrons. Web group 2 elements are alkaline earth metals in which beryllium, magnesium, calcium, strontium, barium and radium are present when group 2 elements form ions. There are two types of ions.
Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), And Radium (Ra).
X → x⁺ + e⁻. Web test match created by dkmoran terms in this set (47) horizontal row in the periodic table period vertical column in the periodic table group basis for mendeleev's arrangement of. As you know, neutral atoms become ions by losing or by gaining electrons. Web answer they lose 2 electrons.
It Is Formed When An Atom Loses The Electrons.
When atom lose electron positive charge is created on atom. Web when group 2a elements form ions what happens? When group 2a elements form ions, they lose two electrons. Web in any chemical compound, the masses of elements are always in the same proportion by mass.