Xolair Enrollment Form Pdf
Xolair Enrollment Form Pdf - Xolair ® (omalizumab) fax completed form to 866.531.1025. Web 1 of 2 prescription & enrollment form: These instructions are to be used for both dose strengths. Moderate to severe persistent asthma in adults and pediatric patients 6 years of age and older with a positive skin test or in vitro. Web find xolair® (omalizumab) support for our practice, including financial supports, billing and distribution information, office support materials, & patient education resources. Web both the prescriber service form and the patient consent form must be received before xolair access solutions can begin helping your patient. Web patient enrollment and consent form for patients prescribed prxolair® for chronic idiopathic urticaria (ciu), all sections must be completely filled out (please print). Xolair® (omalizumab) fax completed form to 808.650.6487. (1) all of the following: Web please complete the form below to join support for you.
These instructions are to be used for both dose strengths. Patient’s first name last name middle initial date of birth prescriber’s first. Web step 14 “after the injection”) xolair prefilled syringes are available in 2 dose strengths. Web find xolair® (omalizumab) support for our practice, including financial supports, billing and distribution information, office support materials, & patient education resources. Web xolair ® (omalizumab) prescription type: Web 1 of 2 prescription & enrollment form: Xolair ® (omalizumab) fax completed form to 866.531.1025. 150 mg/dose subcutaneously every 4 weeks 300 mg/dose subcutaneously. Blue cross and blue shield of texas. Web download the form you need to enroll in genentech access solutions.
Xolair® (omalizumab) fax completed form to 808.650.6487. Web prescription & enrollment form: Web the xolair recertification reminder program helps eligible patients avoid potential gaps in their xolair therapy due to insurance recertification requirements. Naïve/new start restart continued therapy. 150 mg/dose subcutaneously every 4 weeks 300 mg/dose subcutaneously. Web xolair will be approved based on one of the following criteria: Web xolair enrollment form date: Web patient enrollment and consent form for patients prescribed prxolair® for chronic idiopathic urticaria (ciu), all sections must be completely filled out (please print). Moderate to severe persistent asthma in adults and pediatric patients 6 years of age and older with a positive skin test or in vitro. Web please print and complete the forms below.
SchoolEnrollmentForm.pdf DocDroid
Web patient enrollment and consent form for patients prescribed prxolair® for chronic idiopathic urticaria (ciu), all sections must be completely filled out (please print). Web step 14 “after the injection”) xolair prefilled syringes are available in 2 dose strengths. Web prescription & enrollment form: Web patient enrollment and consent form for patients prescribed prxolair® for moderate to severe allergic asthma.
29 [PDF] XOLAIR APPROVAL FORM FREE PRINTABLE DOCX 2020 ApprovalForm2
Middle initial date of birth prescriber’s. Xolair ® (omalizumab) fax completed form to 866.531.1025. Twelvestone health partners fax referral to: Moderate to severe persistent asthma in adults and pediatric patients 6 years of age and older with a positive skin test or in vitro. Patient’s first name last name middle initial date of birth prescriber’s first.
Sample Ach Enrollment Form Form Resume Examples goVLPd3Vva
150 mg/dose subcutaneously every 4 weeks 300 mg/dose subcutaneously. Xolair ® (omalizumab) fax completed form to 866.531.1025. Moderate to severe persistent asthma in adults and pediatric patients 6 years of age and older with a positive skin test or in vitro. Use this form to enroll patients in xolair. Web find xolair® (omalizumab) support for our practice, including financial supports,.
XOLAIR Dosage & Rx Info Uses, Side Effects The Clinical Advisor
Web 1 of 2 prescription & enrollment form: Web xolair prior authorization request form please complete this entire form and fax it to: Web step 14 “after the injection”) xolair prefilled syringes are available in 2 dose strengths. (a) patient has been established on therapy with xolair for moderate to severe persistent. Web xolair will be approved based on one.
MS Enrollment Form PDF Host
Web xolair prior authorization request form please complete this entire form and fax it to: Web patient enrollment and consent form for patients prescribed prxolair® for moderate to severe allergic asthma (aa), chronic idiopathic urticaria (ciu), or severe chronic. Xolair® (omalizumab) fax completed form to 808.650.6487. Once completed, fax to the number indicated on the form. Web patient enrollment and.
Student Enrollment Form California Edit, Fill, Sign Online Handypdf
Twelvestone health partners fax referral to: Xolair® (omalizumab) fax completed form to 808.650.6487. Web the xolair recertification reminder program helps eligible patients avoid potential gaps in their xolair therapy due to insurance recertification requirements. Web patient enrollment and consent form for patients prescribed prxolair® for chronic idiopathic urticaria (ciu), all sections must be completely filled out (please print). Use this.
Xolair Enrollment Form Enrollment Form
These instructions are to be used for both dose strengths. Web please complete the form below to join support for you. Twelvestone health partners fax referral to: Web xolair® (omalizumab) enrollment form xolair® (omalizumab) enrollment form fax completed form to: Middle initial date of birth prescriber’s.
Vivitrol Enrollment Form Fill Out and Sign Printable PDF Template
Once completed, fax to the number indicated on the form. Web the xolair recertification reminder program helps eligible patients avoid potential gaps in their xolair therapy due to insurance recertification requirements. Moderate to severe persistent asthma in adults and pediatric patients 6 years of age and older with a positive skin test or in vitro. Patient’s first name last name.
Xolair requirement Centre of Excellence in Severe Asthma
Naïve/new start restart continued therapy. Middle initial date of birth prescriber’s. Web xolair prior authorization request form please complete this entire form and fax it to: Xolair® (omalizumab) fax completed form to 808.650.6487. Twelvestone health partners fax referral to:
XOLAIR® (omalizumab) Injection Preparation and Administration
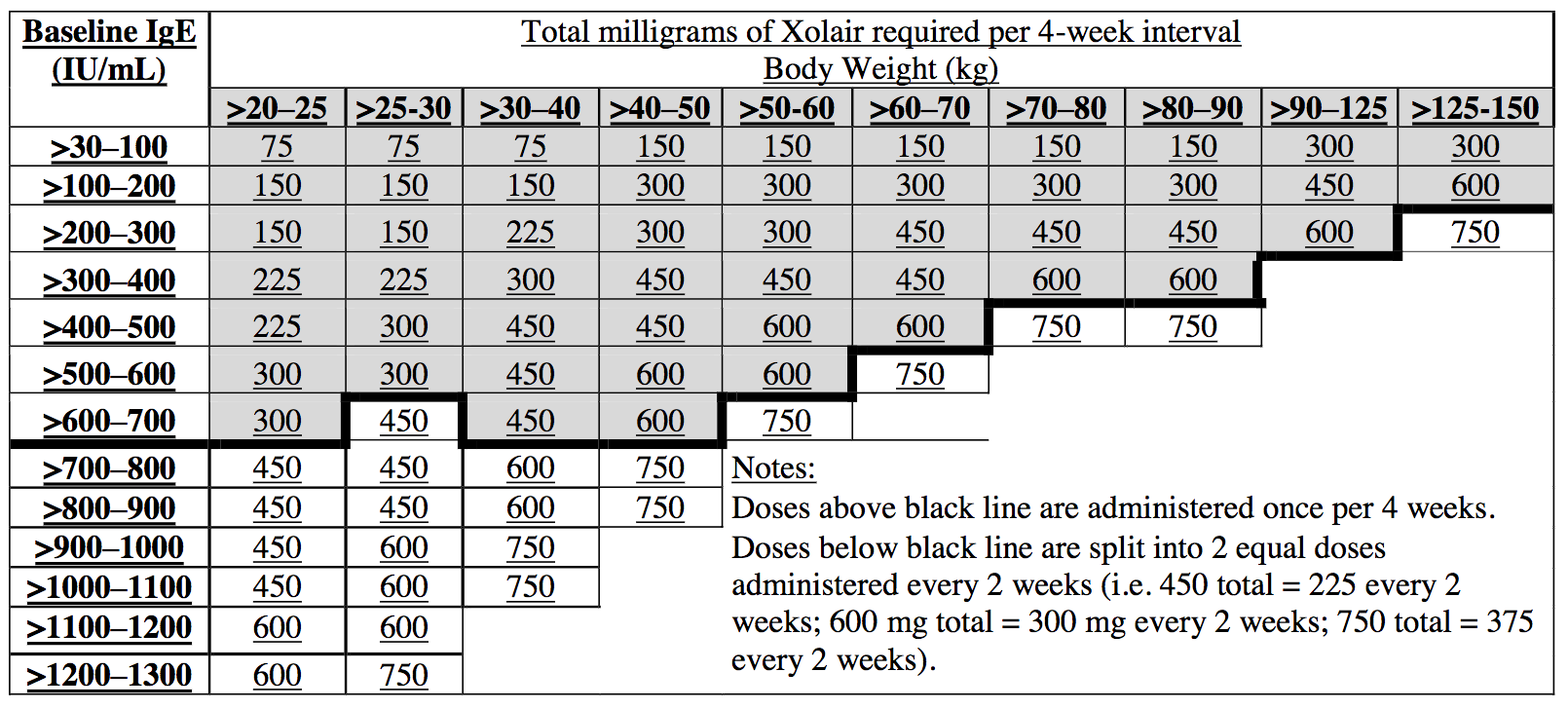
Web 4 prescribing information medication strength/formulation directions quantity/refills xolair® (omalizumab) asthma(dose is dependent on weight and ige. 150 mg/dose subcutaneously every 4 weeks 300 mg/dose subcutaneously. Web step 14 “after the injection”) xolair prefilled syringes are available in 2 dose strengths. Web 1 of 2 prescription & enrollment form: Referral forms for xolair® (omalizumab):
Blue Cross And Blue Shield Of Texas.
These instructions are to be used for both dose strengths. Web find xolair® (omalizumab) support for our practice, including financial supports, billing and distribution information, office support materials, & patient education resources. Web xolair ® (omalizumab) prescription type: Web 4 prescribing information medication strength/formulation directions quantity/refills xolair® (omalizumab) asthma(dose is dependent on weight and ige.
Web Prescription & Enrollment Form:
Web both the prescriber service form and the patient consent form must be received before xolair access solutions can begin helping your patient. Use this form to enroll patients in xolair. Web 1 of 2 prescription & enrollment form: Web xolair enrollment form date:
Referral Forms For Xolair® (Omalizumab):
Web xolair will be approved based on one of the following criteria: Start enrollment with the patient consent form to get started, fill out the patient consent form. Moderate to severe persistent asthma in adults and pediatric patients 6 years of age and older with a positive skin test or in vitro. 150 mg/dose subcutaneously every 4 weeks 300 mg/dose subcutaneously.
(1) All Of The Following:
Before providing your information, let’s confirm that you are eligible to join today. (a) patient has been established on therapy with xolair for moderate to severe persistent. Naïve/new start restart continued therapy. Web patient enrollment and consent form for patients prescribed prxolair® for chronic idiopathic urticaria (ciu), all sections must be completely filled out (please print).

![29 [PDF] XOLAIR APPROVAL FORM FREE PRINTABLE DOCX 2020 ApprovalForm2](https://data.formsbank.com/pdf_docs_html/123/1235/123591/page_1_thumb_big.png)